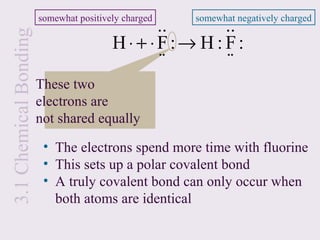
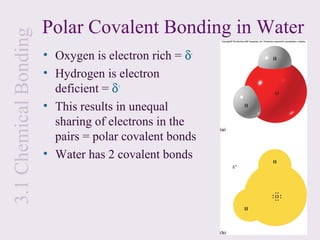
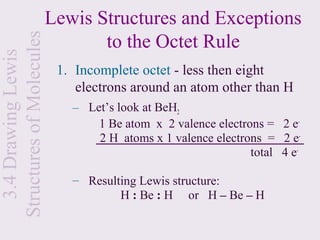
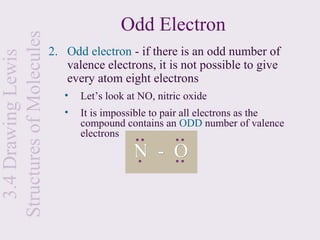
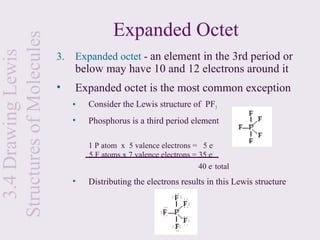
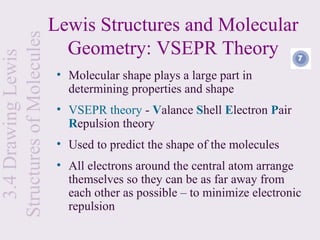
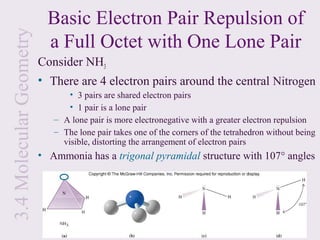
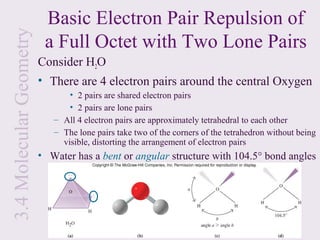
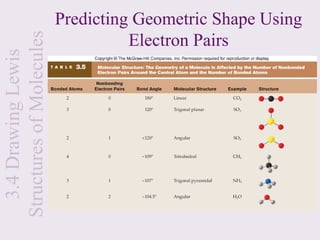
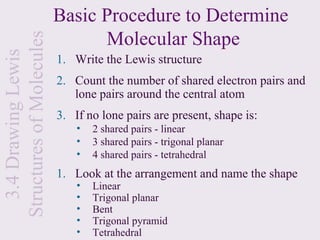
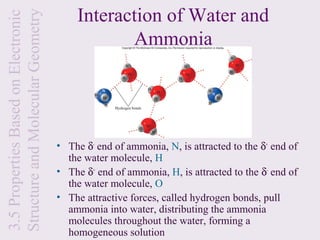
This document provides an overview of chemical bonding and the properties of ionic and covalent compounds. It discusses the following key points:
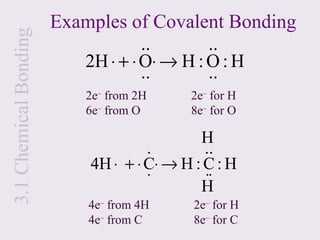
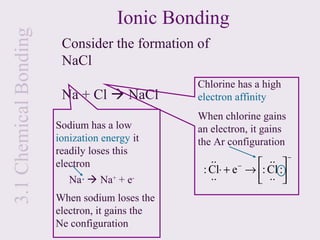
1. Chemical bonds form due to the attraction between atoms and involve the transfer or sharing of valence electrons. Ionic bonds form through electron transfer between metals and nonmetals, while covalent bonds involve electron sharing.
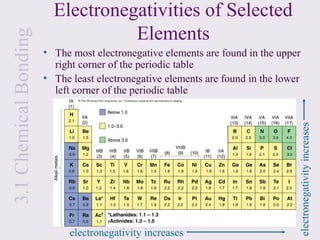
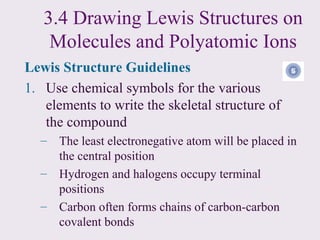
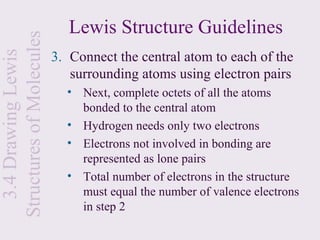
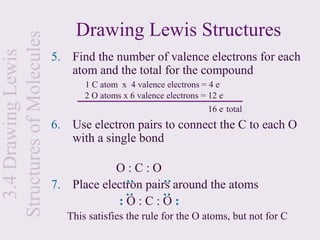
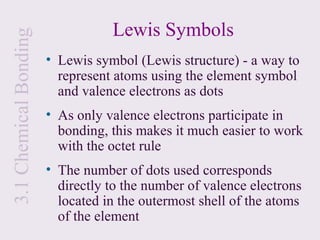
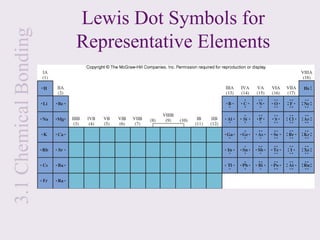
2. Lewis symbols represent atoms and their valence electrons and are used to predict bonding patterns. Electronegativity determines bond polarity.
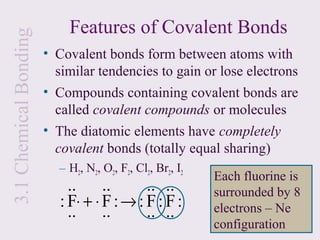
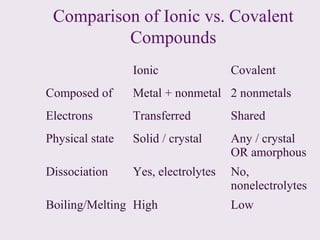
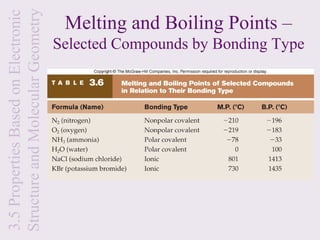
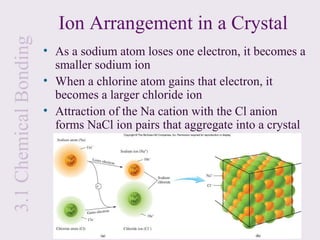
3. Ionic compounds have high melting and boiling points due to strong electrostatic attractions in the crystal lattice. Covalent compounds can be solids, liquids or gases.





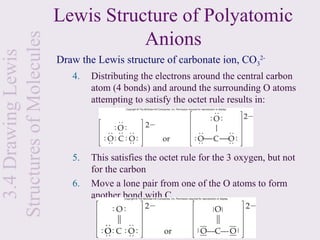
![3.1 Chemical Bonding Covalent Bonding
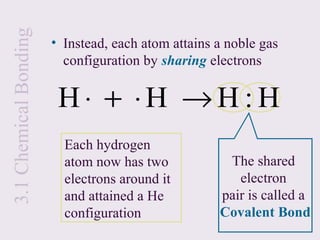
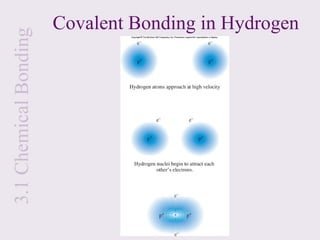
Let’s look at the formation of H2:
H + H H2
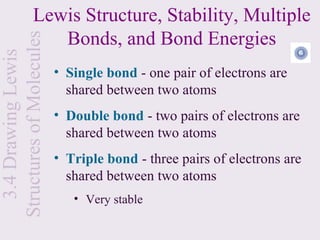
• Each hydrogen has one electron in its valance
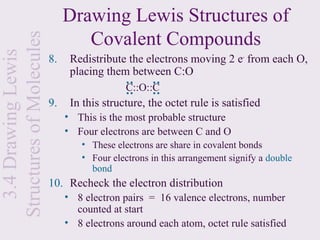
shell
• If it were an ionic bond it would look like this:
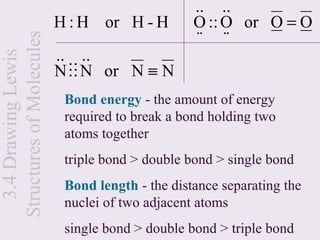
H ⋅ + H ⋅ → H + [ H :]
+ −
• However, both hydrogen atoms have an equal
tendency to gain or lose electrons
• Electron transfer from one H to another usually
will not occur under normal conditions](https://image.slidesharecdn.com/mecchapter3-120815080608-phpapp01/85/Me-cchapter-3-11-320.jpg)