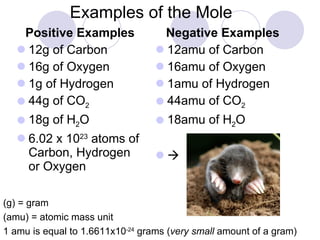
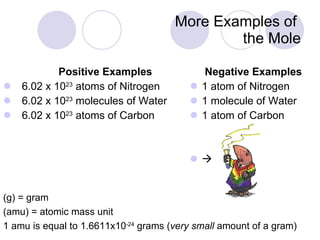
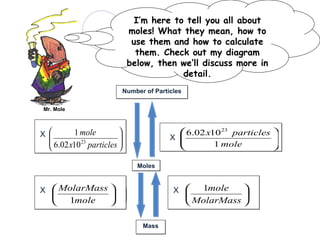
The document provides an introduction to the concept of the mole in chemistry, defining it as a constant equivalent to 6.02 x 10^23 particles and linking the mass of atoms in atomic mass units (amu) to grams. It compares positive and negative examples of the mole to illustrate its characteristics and encourages critical thinking through comparison problems related to atomic masses and quantities. The importance of Avogadro's number in understanding moles is emphasized, along with practical applications in calculating quantities in various substances.