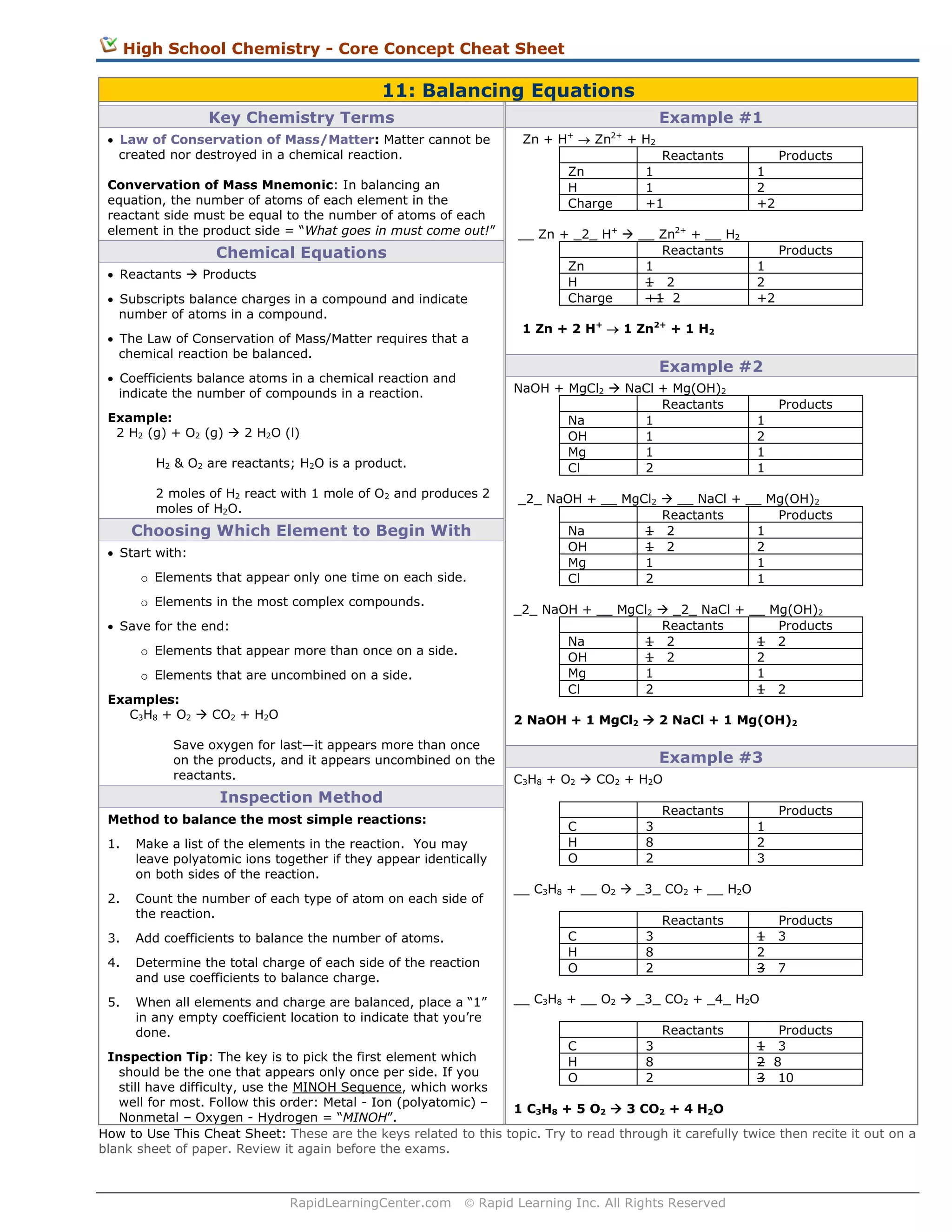
This document provides a summary of key concepts for balancing chemical equations:
1) It outlines the law of conservation of mass which states that matter is neither created nor destroyed in a chemical reaction, so the number of atoms on each side must be equal.
2) It explains how to balance chemical equations using coefficients in front of elements and compounds to make the number of each type of atom equal on both sides.
3) Examples are provided to demonstrate how to systematically balance simple chemical equations by choosing which element to start with and using an inspection method to equalize atoms on each side.