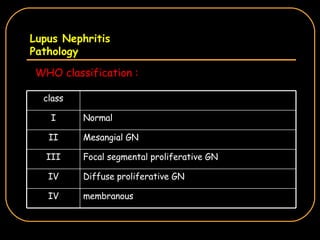
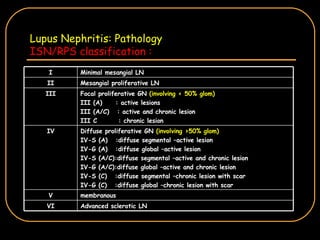
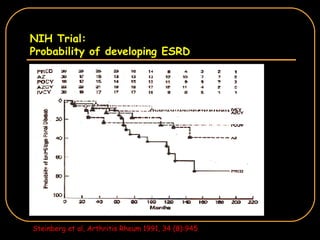
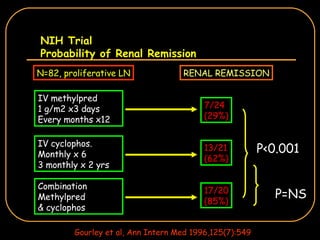
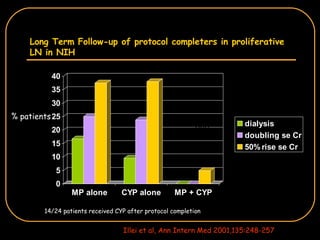
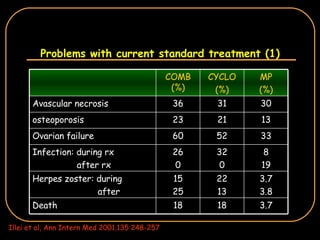
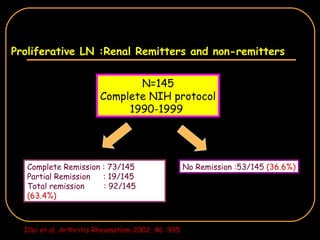
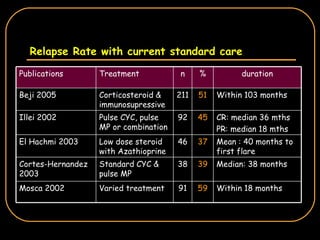
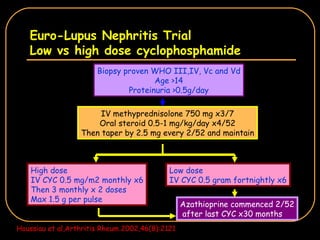
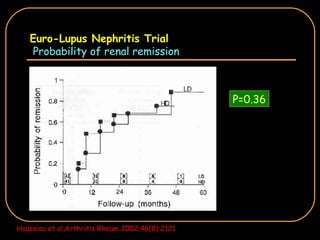
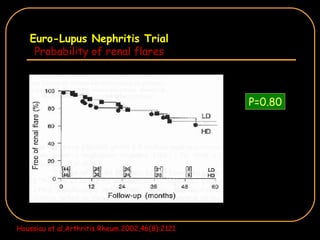
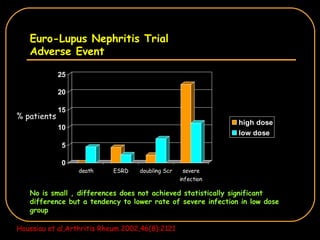
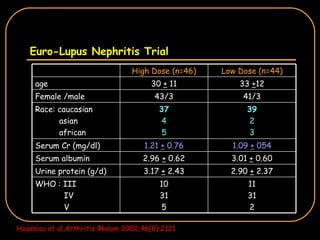
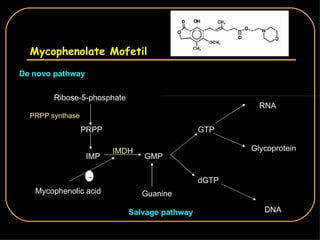
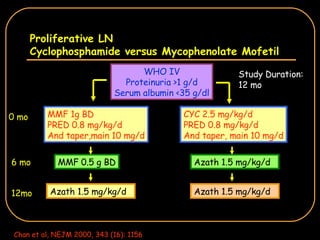
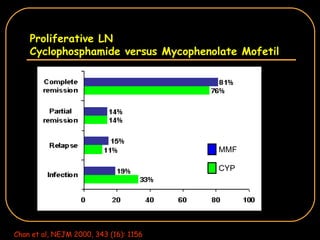
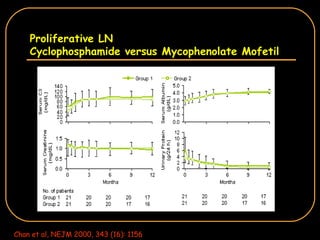
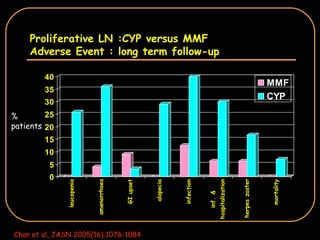
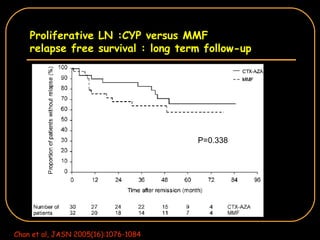
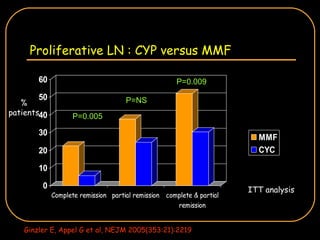
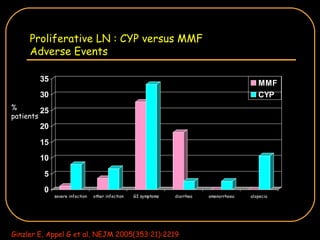
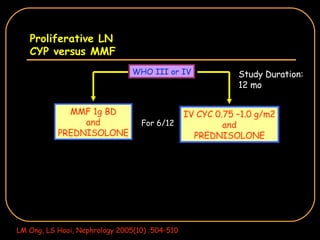
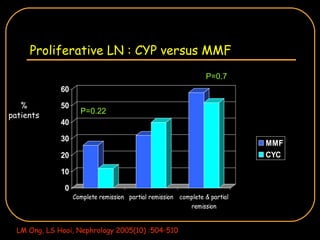
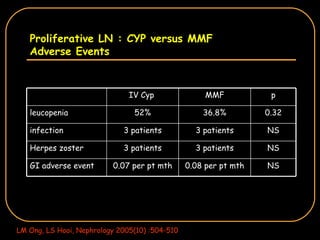
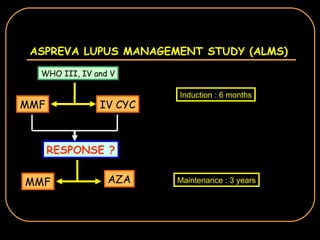
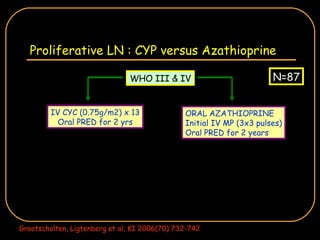
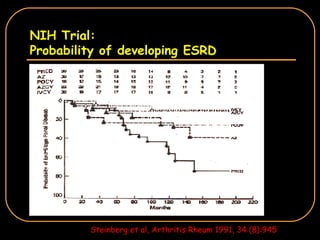
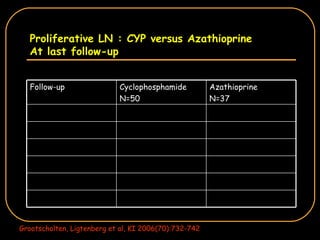
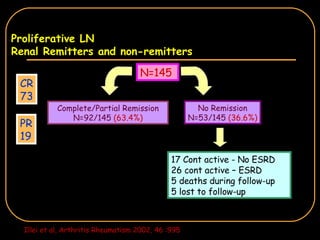
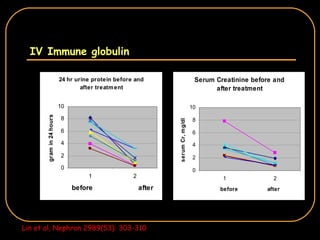
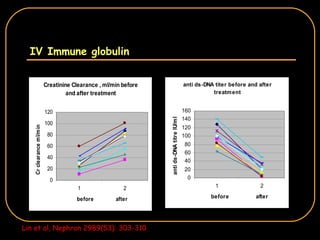
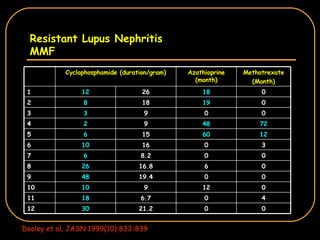
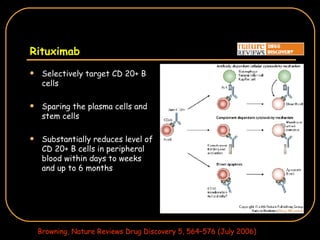
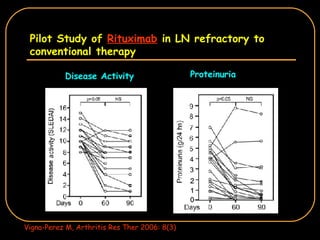
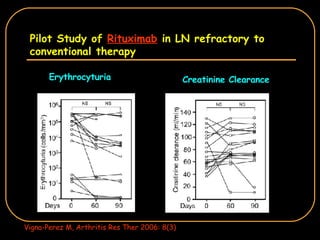
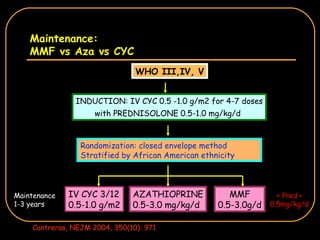
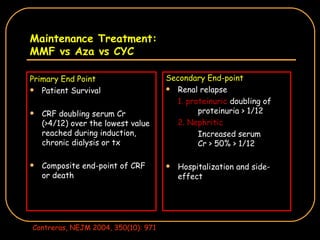
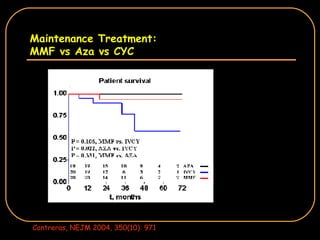
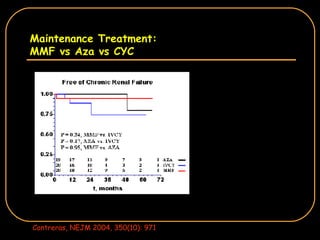
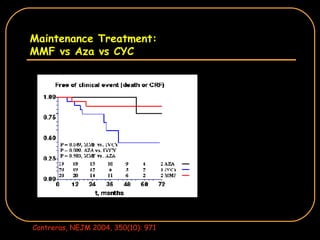
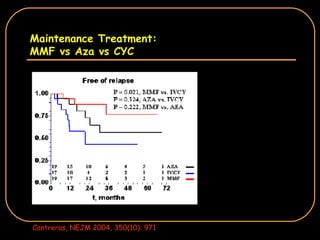
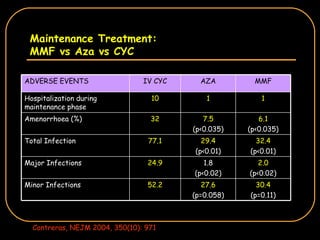
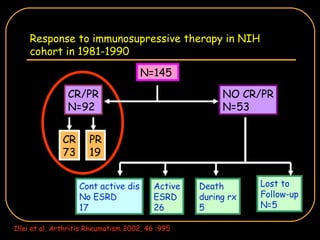
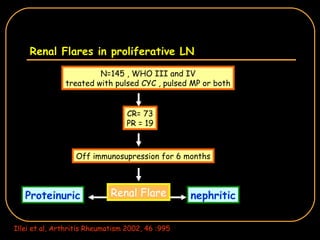
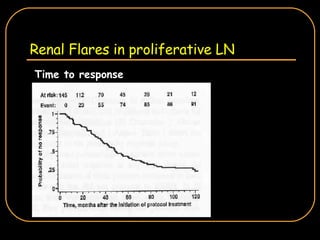
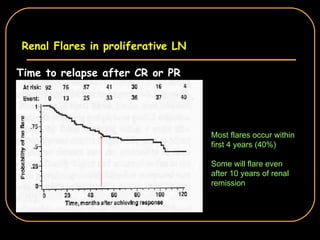
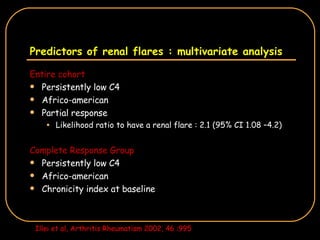
The document discusses treatment options for lupus nephritis, specifically comparing cyclophosphamide to alternative therapies. It finds that while cyclophosphamide is effective for inducing remission, it has significant toxicity risks. Studies show mycophenolate mofetil and low-dose cyclophosphamide have similar efficacy with fewer side effects. Many patients also experience treatment failure or relapse even with standard therapies, highlighting the need for improved maintenance regimens and new treatment agents.