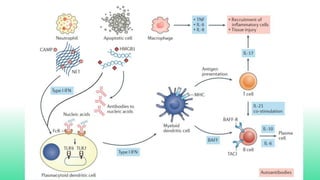
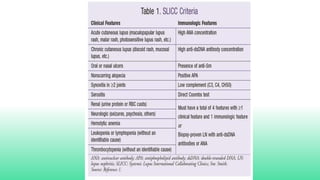
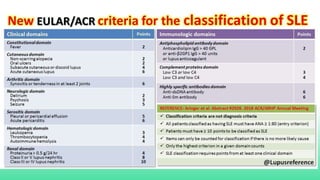
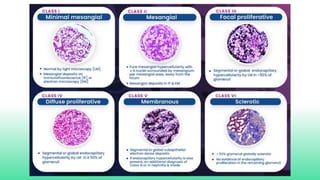
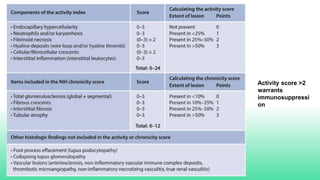
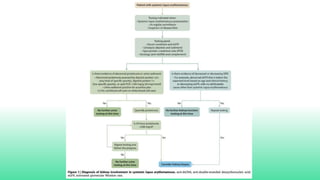
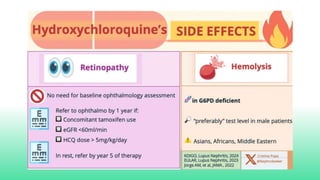
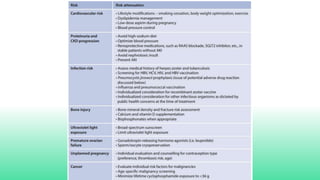
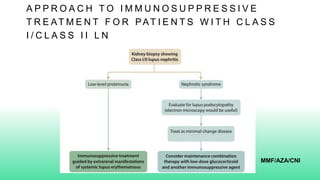
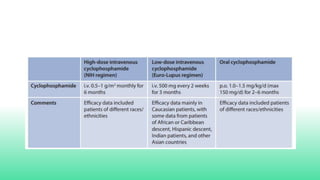
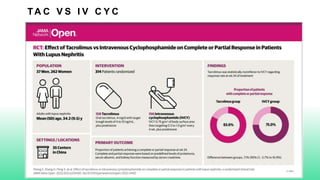
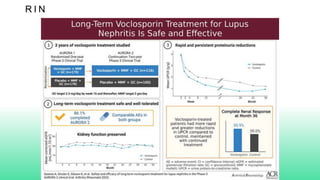
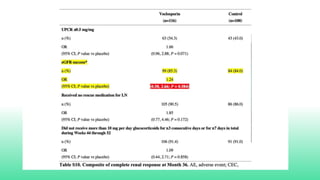
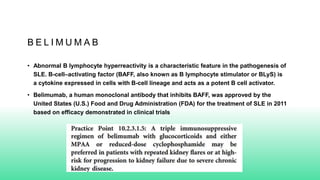
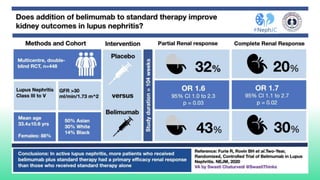
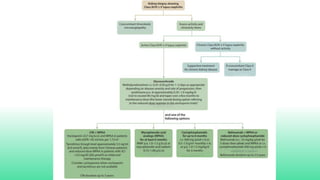
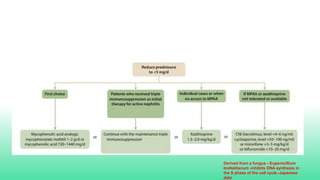
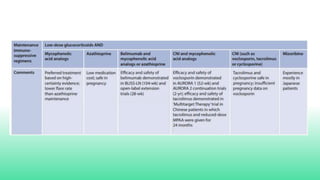
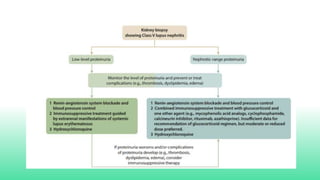
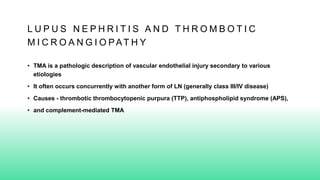
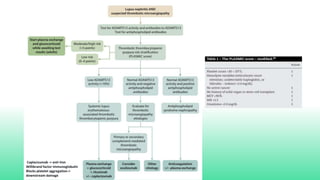
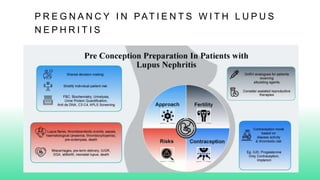
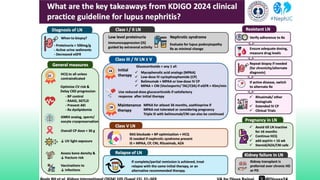
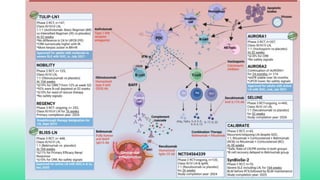
The 2024 guidelines for lupus nephritis (LN) emphasize the need for early diagnosis via kidney biopsy and highlight treatment strategies based on histology and clinical parameters to preserve kidney function. Immunosuppressive therapies are recommended for managing different classes of LN, with considerations for fertility, potential drug toxicities, and response monitoring. Special recommendations address treatment during pregnancy and in pediatric patients, underscoring the continuity of care needed in managing lupus nephritis, especially in cases of relapse or kidney failure.