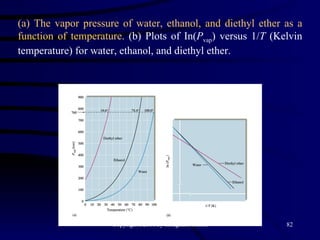
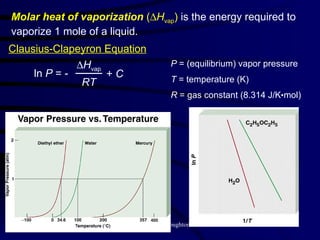
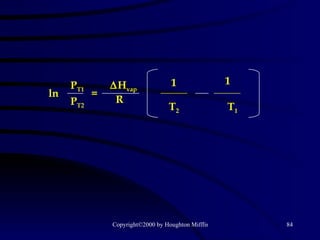
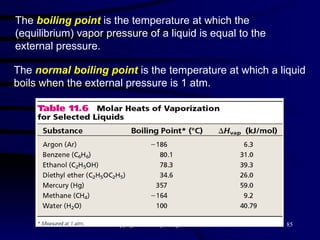
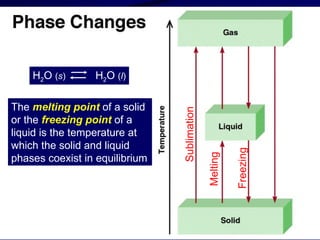
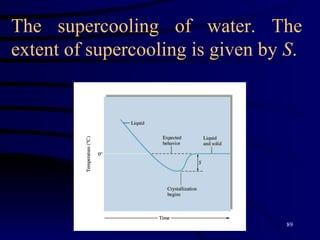
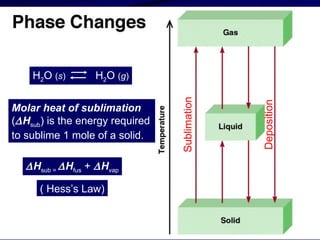
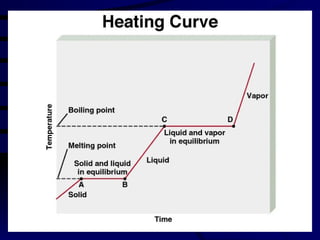
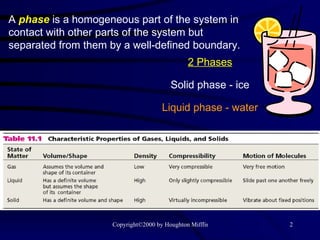
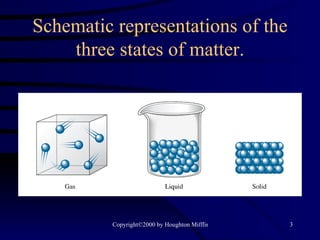
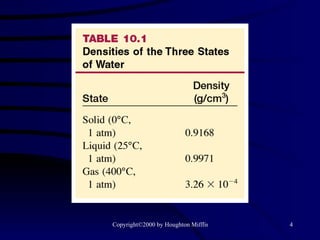
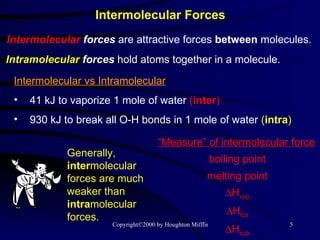
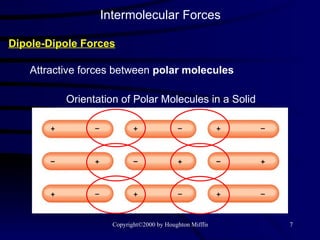
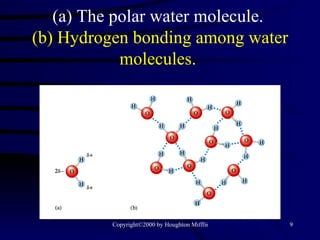
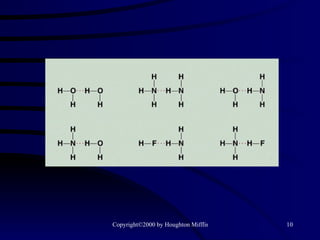
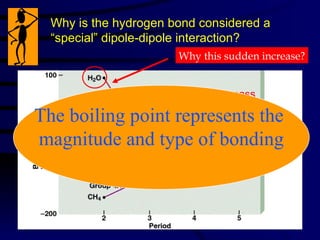
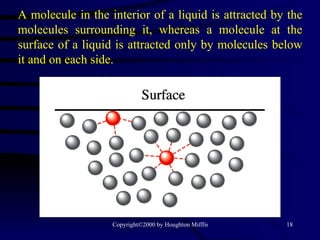
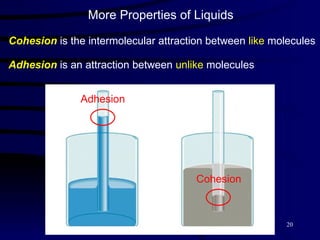
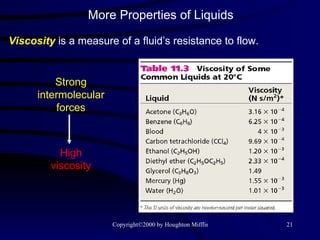
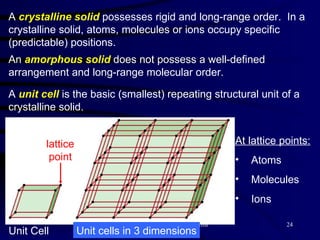
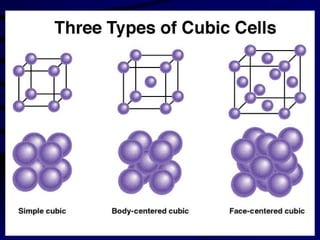
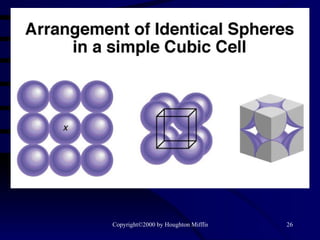
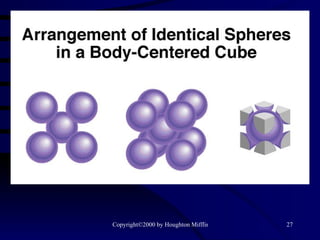
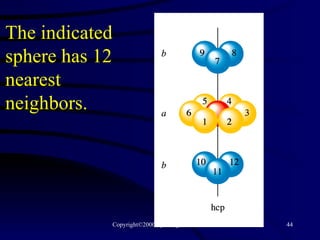
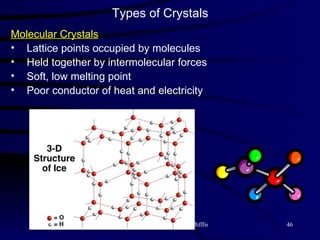
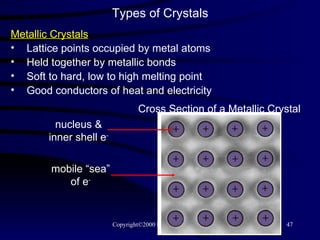
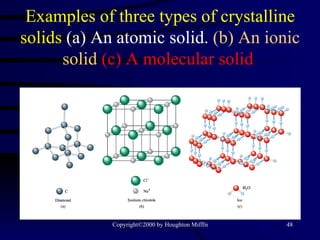
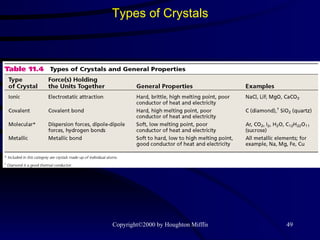
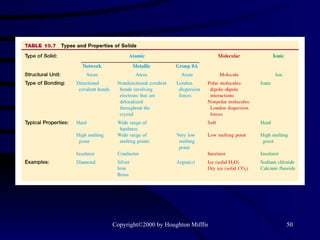
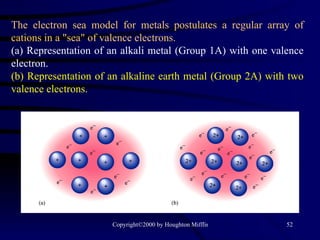
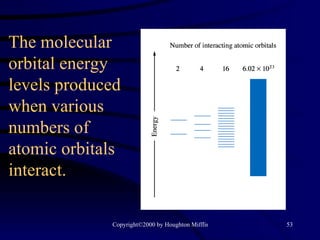
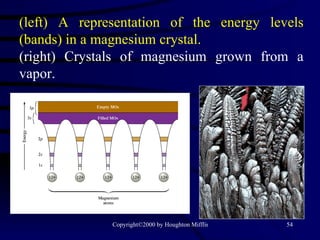
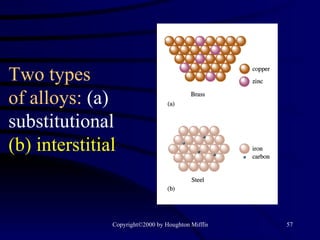
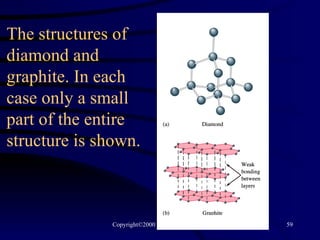
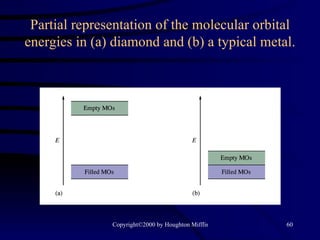
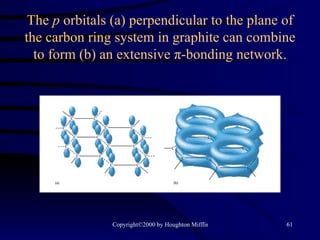
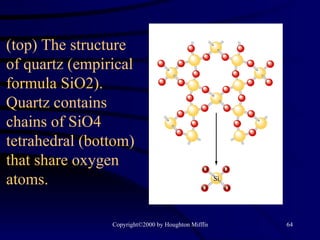
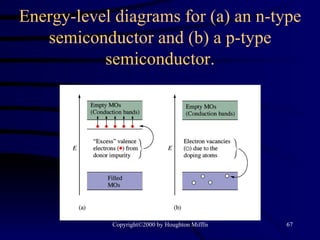
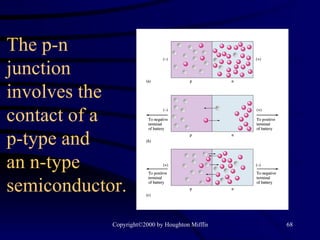
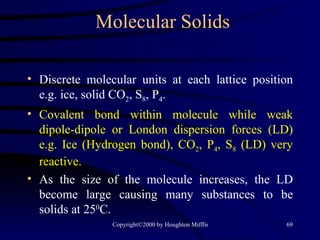
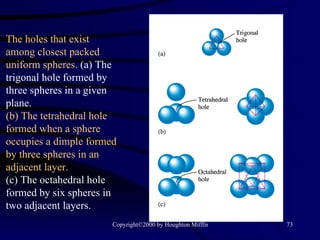
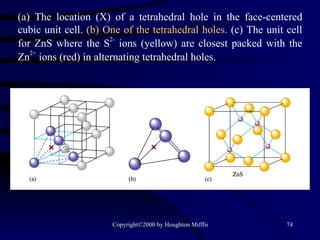
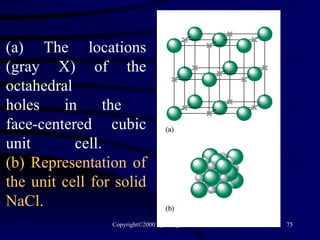
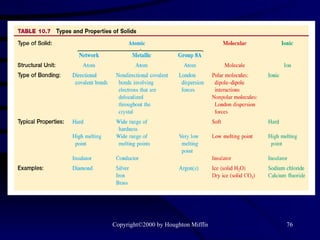
This document discusses different states of matter and properties of liquids and solids. It defines key terms like phases, intermolecular forces, and boiling point. It describes different types of solids like ionic, molecular, metallic and network solids. It also discusses properties of liquids like surface tension, capillary action, and viscosity.






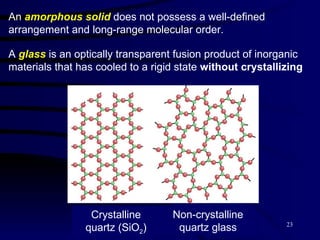
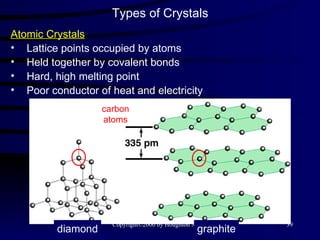
![Types of Solids Crystalline Solids : highly regular arrangement of their components [ table salt (NaCl), pyrite (FeS 2 ) ]. Amorphous solids : considerable disorder in their structures ( glass ).](https://image.slidesharecdn.com/liquids-and-solids-19260/85/Liquids-and-Solids-22-320.jpg)
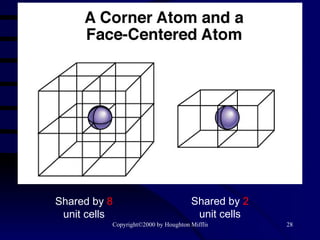
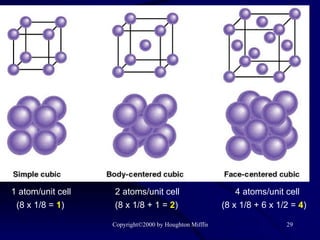
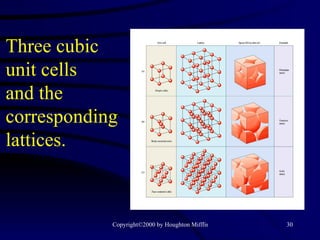
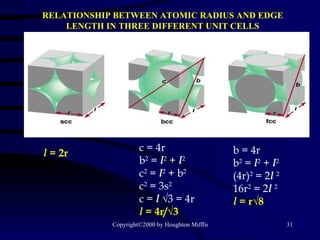


















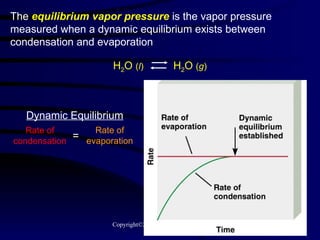
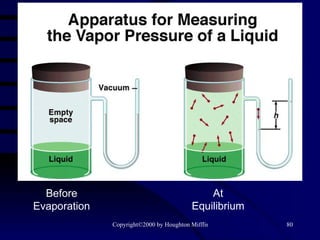
![(a) The vapor pressure of a liquid can be measured easily using a simple barometer of the type shown here. (b) The three liquids, water, ethanol (C 2 H 5 OH), and diethyl ether [(C 2 H 5 ) 2 O], have quite different vapor pressures.](https://image.slidesharecdn.com/liquids-and-solids-19260/85/Liquids-and-Solids-81-320.jpg)