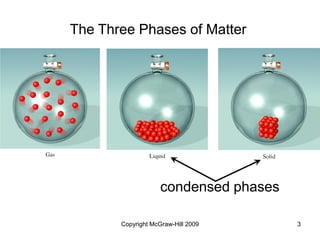
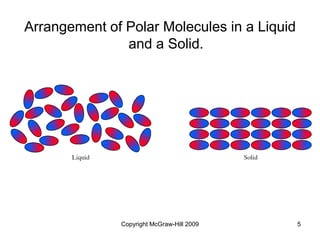
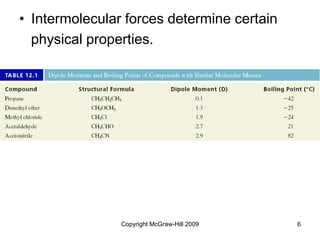
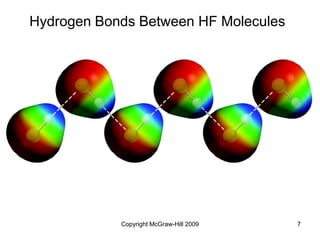
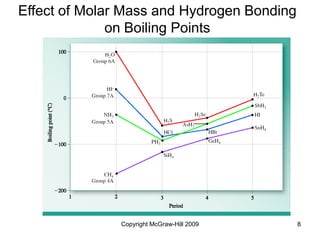
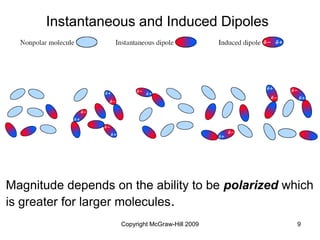
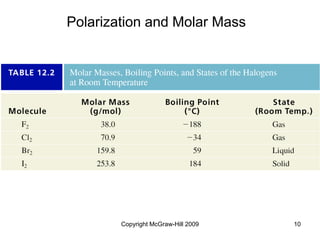
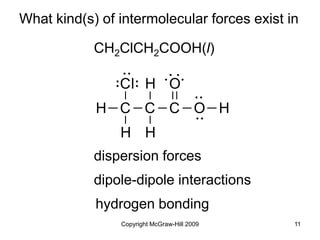
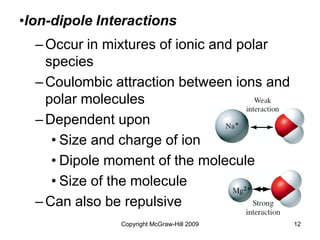
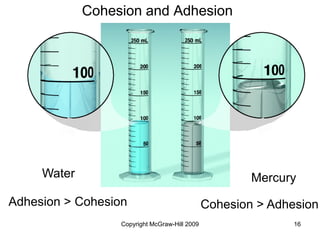
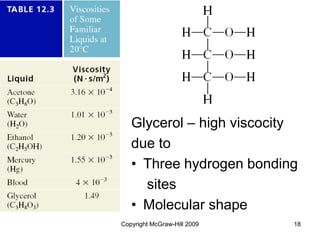
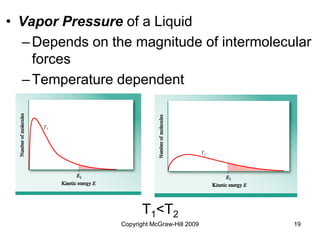
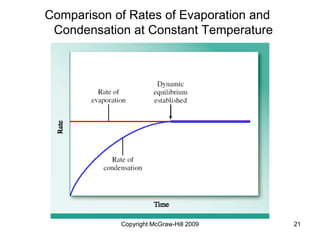
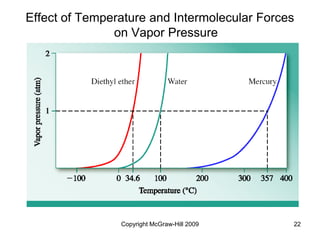
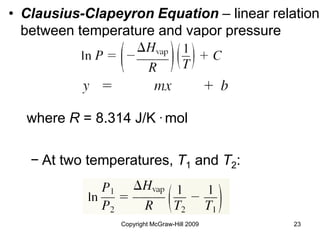
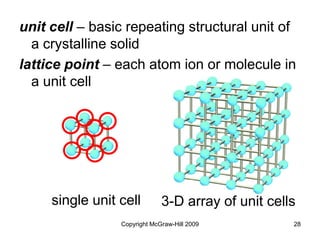
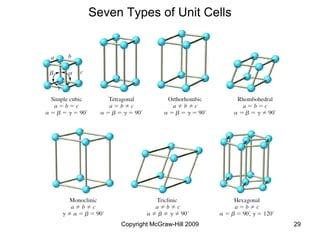
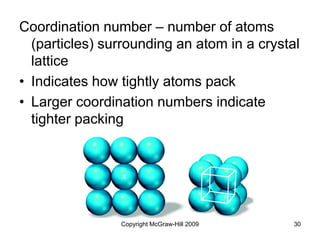
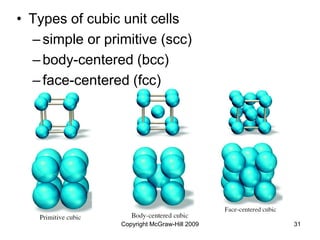
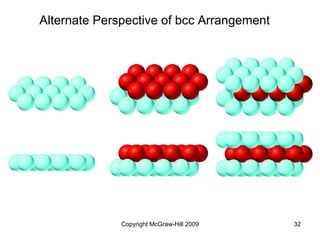
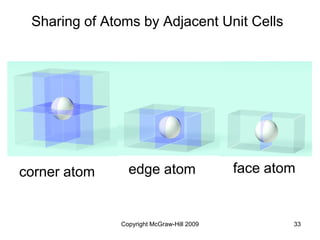
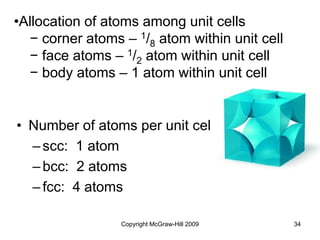
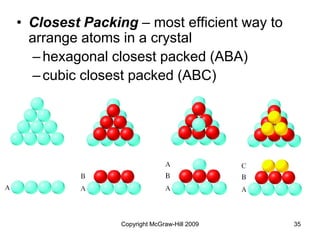
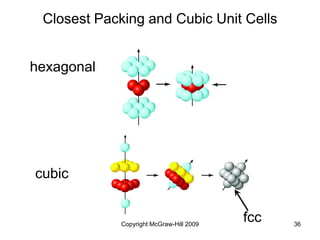
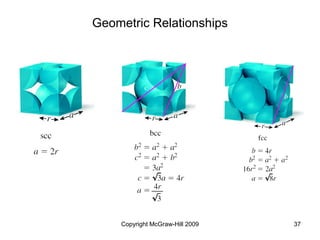
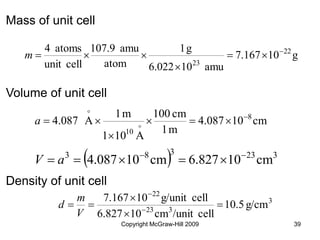
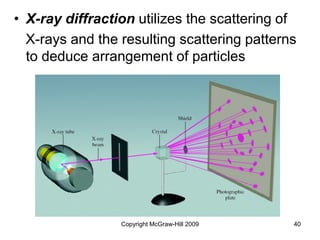
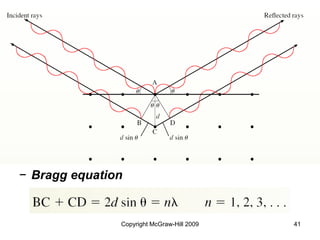
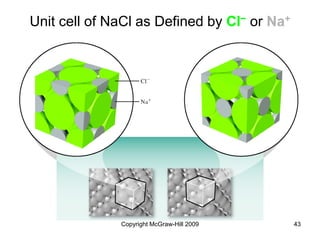
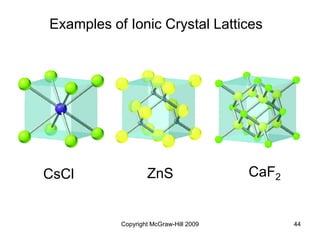
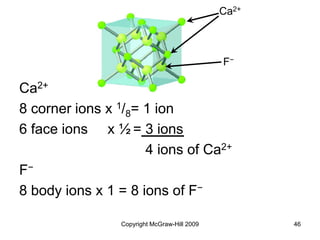
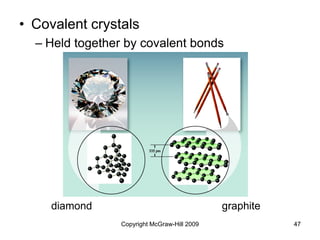
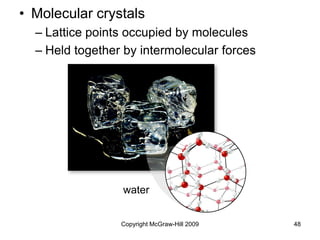
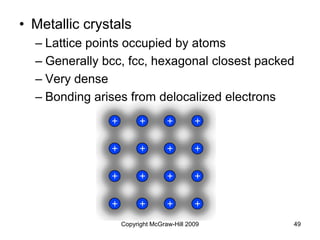
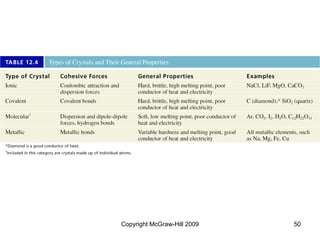
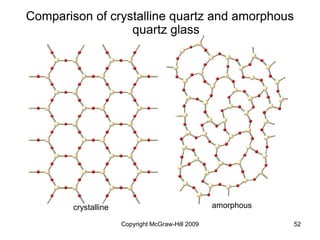
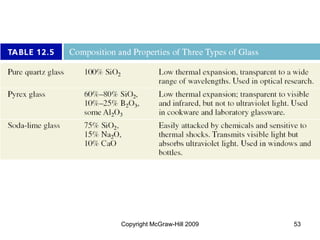
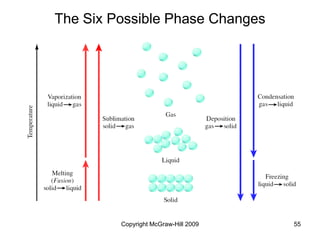
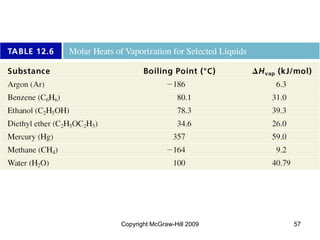
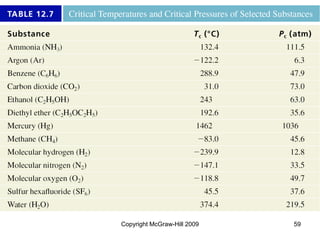
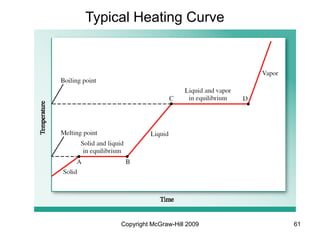
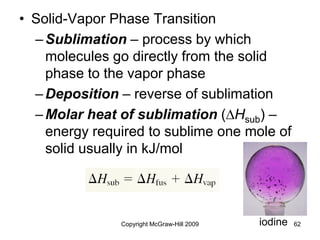
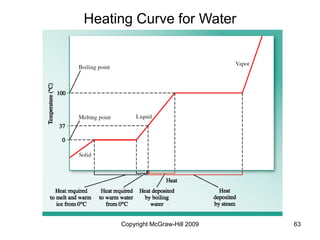
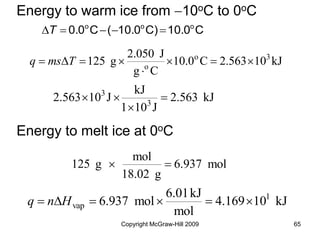
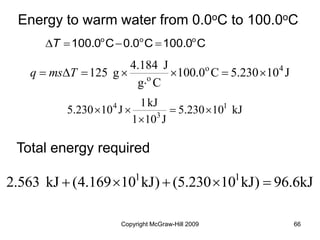
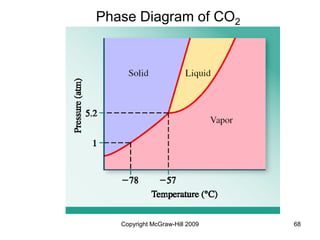
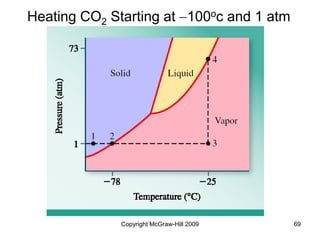
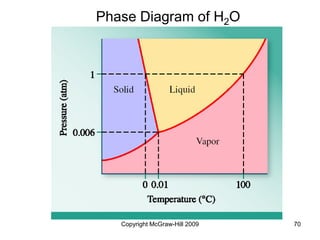
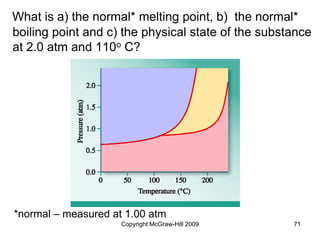
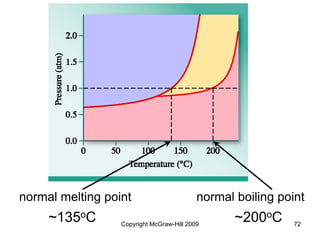
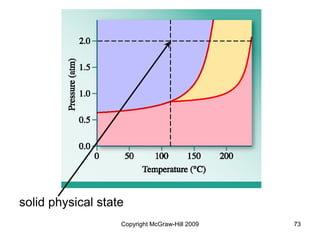
This document discusses intermolecular forces and the physical properties of liquids and solids. It covers topics such as the different types of intermolecular forces, how these forces determine properties of liquids like viscosity and vapor pressure, crystal structure of solids, and phase changes between the different states of matter. The key points are that intermolecular forces are weaker than ionic or covalent bonds but still significantly impact properties, liquids and solids exist as a result of these intermolecular interactions, and phase changes occur with the addition or removal of heat to overcome these attractive forces.