This document discusses chemical bonding concepts including:
- Valence electrons and Lewis dot structures for representative elements.
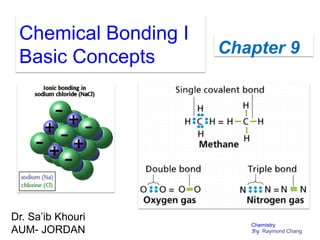
- Ionic bonding formation through electron transfer between atoms.
- Covalent bonding through electron sharing between atoms.
- Factors that influence lattice energy of ionic compounds such as charge and ion size.
- Drawing Lewis structures and evaluating formal charges to determine most likely structures.


![4
Li + F Li+
F -
The Ionic Bond
1s22s11s22s22p5 1s21s22s22p6[He][Ne]
Li Li+ + e-
e- + F F -
F -Li+ + Li+
F -
LiF
Ionic bond: the electrostatic force that holds ions together in an
ionic compound.](https://image.slidesharecdn.com/ch9chemicalbondingibasicconcepts-180114142003/85/Ch9-chemical-bonding-i-basic-concepts-4-320.jpg)








































