This document discusses different types of solids and their properties. It begins by introducing the three states of matter and describing how atoms in solids are held together more strongly than in gases and liquids.
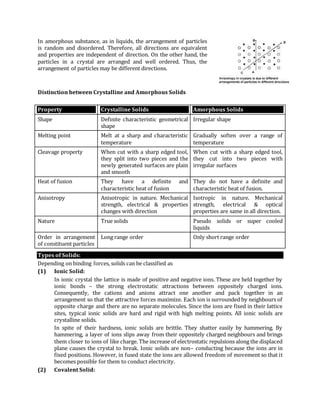
It then summarizes the two main types of solids - amorphous and crystalline. Amorphous solids like glass have irregular atomic arrangements while crystalline solids have orderly, repeating patterns. Crystalline solids can further be classified based on the bonding forces between their constituent particles as ionic, covalent, molecular or metallic. Each type of bonding gives rise to distinct physical properties.
The document also describes space lattices and unit cells, which are the repeating arrangements of atoms that define crystalline structure. There






![5. b 6. b 7. a
Packing of Spheres in Solids
In the formation of crystals, the constituent particles (atoms, ions or molecules) get closely package
together. The closely packed arrangement is that in which maximum available space is occupied
leaving minimum vacant space. This corresponds to a state of maximum possible density. The
closer the packing, the greater in the stability of the packed system.
As the constituent particle of crystals may be of varying shapes and, therefore, the mode of closest
packing of particles will vary according to their shapes and sizes. However, for understanding we
can use identical hard spheres of equal size to represent atoms in a metal in terms of closest
packing of identical spheres.
(A) Close Packing in One Dimension
There is only way of arranging spheres in one dimensional close packet structure in which
the spheres are placed in a horizontal row touching each other. This is shown in figure.
Close packing of spheres in one dimension.
As can be seen, in the arrangement each sphere is in contact with two of its negihbours. The
number of spheres which are touching a given sphere is called its co–ordination
number. Thus, in one dimensional close packed arrangement, the coordination number is
2.
(B) Close Packing in Two Dimensions
Two dimensional close packet structure can be generated by placing the rows of close
packed spheres. The rows can be combined in the following two ways with respect to the
first row to build a crystal plane:
i) The spheres are packed in such a way that the rows have a horizontal as well as
vertical alignment. In this arrangement, the spheres of second row are exactly above
those of the first row. The second row is exactly same as the first one. This type of
packing is also called square close packing in two dimensions.
Square close packing occupies 52.4% of available space [Co. No. 4]
ii) The spheres are packed in such a way that the spheres in the second row are placed
in the depression between the spheres of the first row. Similarly, the spheres in the
third row and placed in the depressions between the spheres of the second row and
so on. In this arrangement, the second row is different from the first row. But the
spheres in third row are aligned with those of the first row. Similarly, the spheres of
fourth row are aligned with those of second row. If the arrangement of spheres in
first row is called ‘A’ type, the one in the second row is different and may be called
‘B’ type. Now, the arrangement of spheres of third row is same as that of first row,
and therefore, it is also called ‘A’ type. Similarly, fourth row is called ‘B’ type. Hence
the arrangement is called ABAB …… type. This type of arrangement is called
hexagonal close packing of spheres in two dimensions and is shown in figure.](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-9-320.jpg)



![3. Co–ordination number 12
4. Transition metal forms FCC unit cell
(vi) Cubic is the most symmetrical while triclinic is the most unsymmetrical system.
(vii).
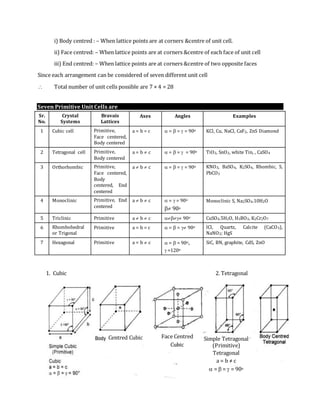
Sr.
No.
Structure Type of packing Stacking pattern Coordinatio
n number
Space
used
(%)
Unit cell
(a) Simple
cubic
Square close
packing
AAA … 6 52 Primitive cubic
(b) Body
centred
cubic
Square close
packing
ABAB….. 8 68 Body centred
cubic
(c) Hexagonal
close
packed
Hexagonal ABAB… 12 74 (Non–cubic)
(d) Cubic close
packed
Hexagonal ABCABC… 12 74 Face centred cubic
(viii) In FCC or CCP there are three different layers which are constantly repeated in three
dimensions. In HCP every first & third layers are same and in ccp every first & fourth layers
are same.
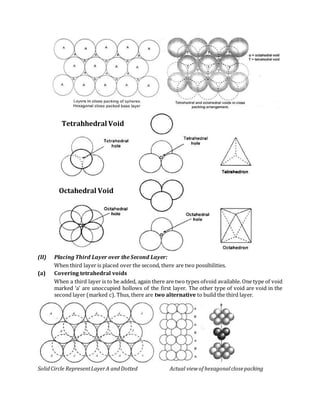
Between A & B (in close packing) the Octahedral & tetrahedral voids are located.
If we place atoms of 3rd layer over the tetrahedral void, the layer A will be repeated, hence
ABAB….. structure or HCP will be formed.
If we place atoms of third layer over Octahedral void, then FCC will be generated as third
layer will be different layer & it is ABCABC ….. type.
The number of nearest neighbours for any atom, molecule or ion in a space lattice is called
the coordination number.
Type of Cubic Unit Cell & Packing Fraction
1. Simple or Primitive Cubic Unit Cell:
A cubic unit cell is said to be primitive if
(a) All the eight corners of the cube are occupied by the same
atoms & will touch each other.
(b) There is no such atoms present any where in the cube except
corners.
(c) Contribution of each atom or sphere for one unit cell is 1/8 of the volume of each sphere.
(d) Therefore, number of effective atoms in a unit cell. [Rank of unit cell] is one
Z = no. of corner atoms (nc) × contribution of each atoms = 1
8
1
8
(e) Each face made by four corner atoms are in contact with each other.
(f) Relation between edge length (a) of cube and radius of a sphere (r).
a = 2r](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-14-320.jpg)

![AD2 = 222
a3aa2
AD = a.3 r = radius of atom and a = edge length
Body diagonal of cube = a3r4
d = 2r
2
a3
dor
4
a3
r
Packing fraction (PF) =
cellunitofVolume
cellunittheinspheresthebyoccupiedVolume
Volume of unit cell =
3
r4
a
3
r4
a
3
3
And volume occupied by the spheres in the unit cell
= Z × volume of a sphere
= ]bccfor2Z[r
3
4
2 3
= %02.686802.0
3
r4
r
3
4
2
3
3
Void fraction = 0.32 = 32%
Case – II
When the body centred atom and the corner atoms are different
)r2r2(a3AB
…… (1)
= 3
3
a
3
c
a
r
3
4
r
3
4
rc = radius of cation, ra = radius of anion
Limiting condition or Ideal Condition:
2ra = a …… (ii)
The limiting condition corresponds to the maximum radius of the anion. Corner atoms are making a
contact along the edge of a cube. So, 2ra max = a
3 a = 2rc + 2ra Substitute volume of ‘a’ from eq. (ii)
3 (2ra) = 2rc + 2r a
2rc = 2ra 13
ac r13r
732.0
r
r
a
c
It is the minimum possible radius ratio in BCC](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-16-320.jpg)

![Next nearest
neighbour [12]
Packing fraction (PF) =
cellunitofVolume
cellunittheinspheresthebyoccupiedVolume
=
7406.0
r22
r
3
4
4
3
3
= 74.06% space is occupied & 26% space is unoccupied
So FCC is also known as cubic closed packing (CCP)
Case II: The face centred atoms and the corner atoms are different
There are 3 face centred atoms (cation) and 1 corner (anion) atom in 1 unit
Packing Fraction = 3
3
a
3
c
a
r
3
4
1r
3
4
3
Limiting or Ideal Conditions: If corner atoms are bigger & are touching
ac r2r2a2AB …. (i)
a = edge length
Corner atoms are making contact along the edge of the cubes
2ra = a …. (ii)
On substituting maximum radius value of anion (r–) in (i)
2 (2ra) = 2rc + 2ra 2rc = 2 (2ra) – 2ra 2rc = 2ra 12
414.012
r
r
mina
c
It refers to the minimum limiting ratio: 414.0
r
r
a
c
Radius ratio range is equal to 0.414 – 0.732 ( value equal to 0.414 but less than 0.732)
So, packing fraction = 635.0]1)414.0(3[
6
1
r
r
3
6r2
rr3
3
4 3
3
a
c
3
a
3
c
3
c
Co–ordination Number or Number of Nearest Neighbours
The nearest of closest equidistant atoms with respect to a given atom is called its nearest
neighbour. The number of nearest neighbor is called co–ordination number. Next nearest
neighbor represents the next close equidistant atom w.r.t a given atom.
(a) Simple cubic unit cell:
Number of Nearest neighbouris six& next nearest neighbour is 12.
Distance between nearest neighbour = a
Distance between next nearest neighbor = a 2](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-18-320.jpg)
![One corner is shared by 12 faces. The atoms meets the next nearest neighbor along the face
diagonal of each face. So the no. of next nearest neighbor will be 12(Number of atom in
each xy; yz; zx plane will be 4]
(b) Body centred Cubic unit cell
(i) The nearest neighbor for Body centred atom will be corner atom.
So the nearest neighbor = 8
distance between neighbouring atom (d) = .a
2
3
(i.e. half of the body diagonal)
(ii) The next nearest neighbour = 6 [i.e. atom of Body
centred of six cube]
Distance between next neighbour = a
(iii) For corner atom, the next nearest neighbour are going to be corner atoms i.e., next nearest
neighbour is 6.
3. Face centred cubic unit cell
No. of Nearest neighbour = 12; No. of next neighbour = 6
With respect to the corner atom, the nearest neighbour is the face centred atom. Number of
nearest neighbours is 12.
Number of Nearest neighbours = 12
Distance between nearest neighbours =
2
a2
(i.e. half of face diagonal)
(i) For corner atom, the next nearest atom is a corner atom i.e. next nearest neighbour = 6
(ii) For face centred atom (A) nearest neighbour atom will be (B) & (C)
(8 faces + 4 corners). So number of nearest neighbour is 12.](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-19-320.jpg)


![In zinc blende; Zn+2 ions are placed in alternate Tetrahedral void & S2– form FCC lattice. One unit cell
is made up of four ZnS units.
In wurtzite S–2 form HCP unit cell i.e. 6 S–2 ions and Zn+2 ions goes in alternate Tetrahedral void i.e. 6
Zn+2 so one unit cell is made up of 6 ZnS units. The radius ratio of guest to host atom remains same
irrespective of the geometry of the unit cell.
P.F.(wurtzite) =
225.0
r
r
voidltetrahedrainas
r224
)rr
3
4
6
a
c
3
a
3
a
3
c
= ]1)225.0[(
23
3
= 0.74 (1.01) = 74.86%
So, the packing fraction of zinc blende &wurtzite will be same, but their formula weight will be
different.
Voids or Interstitial Site
Vacant space in the crystal lattice is called void. It is made up of host atoms (bigger) atoms which is
usually an anion. Small sized atoms are occupied in these voids. Sometimes an impurity atom can be
present in a void, which is called guest atom or foreign atom. The guest atom must make a contact
with host atom, but host atom may or may not make contact with each other. e.g. In II case, we get
the limiting radius ratio, which corresponds to the minimum value
There are four types of void existed:
(a) Triangular (b) Octahedral Voids (c) Tetrahedral Voids (d) Cubic Voids
Triangular Voids
(a) Triangular Voids](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-22-320.jpg)






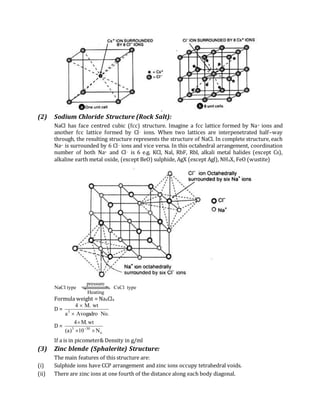
![(iii) Only half of the alternate tetrahedral voids are occupied by Zn2+ ions. If S–2 ions are at lattice
point, there are Zn+2 ion at ¼ of the distance along each body diagonal.
(iv) Coordination no. of Zn2+ ions as well as S2– ions is 4. Thus, this structure has 4 : 4
coordination number.
Here electronegativity difference between Zn and S is
very small i.e., only 0.9 (electronegativity for Zn = 1.6
and S = 2.5), therefore, the bond between Zn and S
has significant covalent character.
Packing fraction of ZnS (Zinc blende):
= 3
3
a
3
c
a
4r
3
4
3
4
=
.]a2r4as[
2
r4
rr
3
16
a3
a
3
a
3
c
012.0)225.0(
392.0)732.0(
071.0)414.0(
3
3
3
=
1
r
r
23
3
a
c
Limiting value (tetrahedral void) = 225.0
r
r
a
c
= 1225.0
23
3
= 0.749 or 74.9%
(4) HCP structure of ZnS (Wurtzite)
It has hexagonal unit cell made up of six S–2 ion at corners, face centre& 3 atom in body centre i.e.
one unit cell has six sulphide ion. Zn+2 ions are placed in alternate tetrahedral void.
(5) Diamond:
Space lattice as ZnS, it has two interpenetrating FCC lattice, every carbon is surrounded by 4 other
carbon atom place at the corner of regular tetrahedron.
Carbon atom occupy corner, face centre as well as in 50% of tetrahedral void
cr2
4
a3
](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-30-320.jpg)
![ Packing fraction = 3
c
3
c
3
r8
r
3
4
8
= 34.
16
3
In diamond only 34% of space is occupied & 66% is empty.
Density of Cubic Unit Cell
Density of cube unit cell = 3
a
atomof.noTotalatomoneofMass
cubeofVolume
cellunitofMass
=
oN
wt.At
Total no. of atom × 3
303
o
3
cm/gm
10)pm(aN
ZM
a
1
No = Avogadro’s number; a = edge length; Z = No. of atoms per unit cell;
d = distance between two neighbouring atoms.
In NaCl type unit cell, d = rc + ra = a/2
In CsCl type d = rc + ra = a
2
3
[2d = Body diagonal]
In ZnS type 4ra = a2 In CaF2 Type 4rc = a2
Theoretical density of solid is that which we are supposed to calculate, assuming crystal to be
perfect or ideal. Where as experimental density is defined for imperfect crystal where defects are
present.
(i) If theoretical density is more it will be a vacancy defect.
(ii) If theoretical density is less than observed density it will be metal excess defect without
anion vacancy.
Assignment – III
Q.1 CsCl has cubic structure. Its density is 4.0g cm–3. What is the distance between Cs+ and Cl–
ions? (At. mass of Cs = 133)
Q.2 An element has a body–centred cubic (bcc) structure with edge of 288 pm. The density of
the element is 7.2g/cm3. How many atoms are present in 208g of the element?
Q.3 KF has NaCl structure. If the distance between K+ and F– is 269 pm, find the density of
KF(NA = 6.02 1023mol–1, atomic masses K = 39, F = 19 amu).
Q.4 Calculate the density of silver which crystallizes in a face–centred cubic structure. The
distance between the nearest silver atoms in this structure is 287 pm. (Molar mass of Ag =
107.87 g
mol–1, NA = 6.02 1023mol–1)
Q.5 The density of a face centred cubic element (atomic mass = 60.2 amu) is 6.25g cm–3.
Calculate the length of the edge of the unit cell.
Q.6 What is the distance between Na+ and Cl– in a NaCl crystal if its density is 2.165g cm–3? NaCl
crystallizes in the fcc lattice.
Q.7 Iron (II) oxide has a cubic structure and each unit cell has side 5 Å. If the density of the oxide
is 4g cm–3, calculate the number of Fe2+ and O2– ions present in each unit cell (Molar mass of
FeO = 72g mol–1, NA = 6.02 1023mol–1)
Q.8 (a) An element crystallizes in BCC structure. The edge length of its unit cell is 288 pm. If the
density of the crystals is 7.2g cm–3, what is the atomic mass of the element?
(b) How many atoms of this element are present in 100gm ?](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-31-320.jpg)
![Q.9 An element (density 6.8g cm–3) occurs in the BCC structure with cell edge of 290 pm.
Calculate the number of atoms present in 200g of the element.
Q.10 Tungten has a density of 19.35g cm–3 and the length of the side of the unit cell is 316 pm.
The unit cell in the most important crystalline form of tungsten in the body centred cubic
unit cell. How many atoms of the element does 50g of the element contain?
Q.11 A compound AB crystallizes in lattice similar to bcc with unit cell edge length of 380 pm.
Calculate
(i) the distance between oppositely charged ions in the lattice
(ii) radius of B– if the radius of A+ is 190 pm
Q.12 An element A crystallizes in fcc structure. 200g of this element has 4.12 1024 atoms. The
density of A is 7.2g cm–3. Calculate the edge length of the unit cell.
Q.13 Sodium crystallizes in the cubic lattice and the edge of the unit cell is 430 pm. Calculate the
number of atoms in a unit cell. [Atomic mass of Na = 23.0 amu, Density of sodium = 0.9623g
cm–3, NA = 6.023 1023mol–1]
Q.14 Calculate the approximate number of unit cells present in 1g of ideal NaCl crystals.
Answers
1. 357 pm 2. 24.17 × 1023 atoms 3. 2.48 gm/cm3
4. 10.71 gm/cm3 5. 4 × 10–8 cm 6. 281 pm
7. 4 8. (a) 51.8 (b) 11.62 × 1023 9. 24.12 × 1023
10. 1.638 × 1023 11. (a) 329 pm (b) 139 pm 12. 299.9 pm
13. 2 14. 2.57 × 1021](https://image.slidesharecdn.com/thesolidstate-parti-151007095002-lva1-app6892/85/The-solid-state-part-i-32-320.jpg)