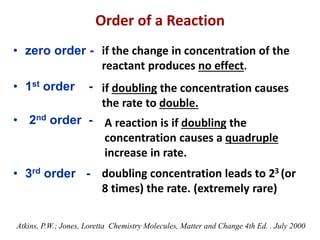
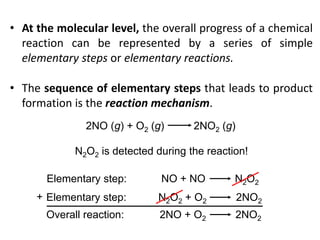
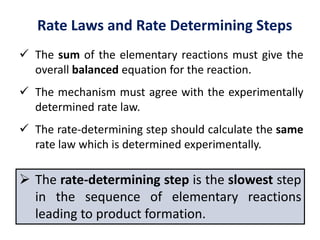
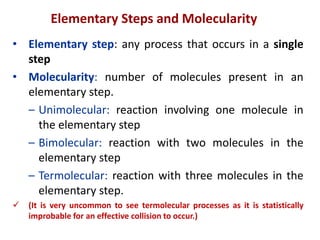
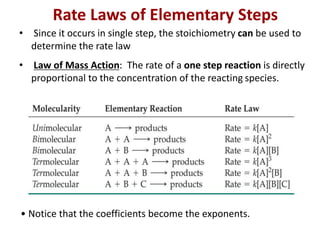
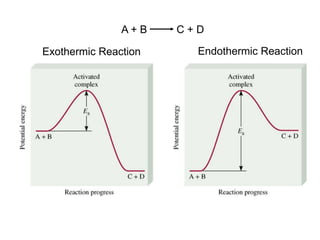
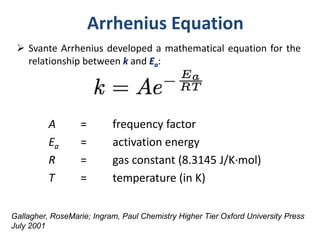
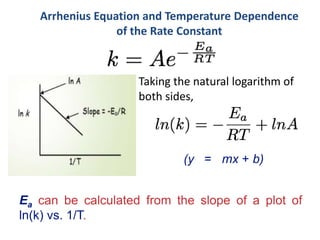
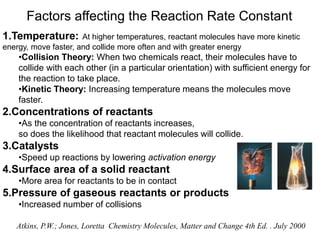
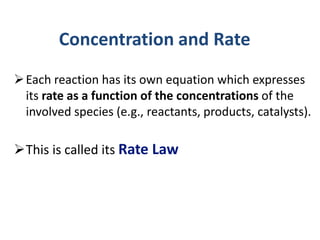
This document provides an introduction to chemical kinetics. It defines chemical kinetics as the study of reaction rates and mechanisms. Reaction rates are measured as changes in concentration over time and can be determined by monitoring reactants or products. The rate of a reaction is affected by factors like temperature, concentration, catalysts, and surface area. Rate laws relate the rate of reaction to concentrations and are determined experimentally. Elementary reactions make up reaction mechanisms, and the slowest step is rate-determining. The Arrhenius equation models the temperature dependence of reaction rates. Hess's law allows calculating enthalpy changes indirectly. Surfactants reduce surface tension and stabilize bubbles and emulsions.

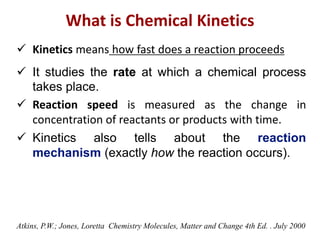
![Rates of Reaction
Rates of reactions can be determined by monitoring the
change in concentration of either reactants or products as a
function of time t.
[A] = concentration of reactant A](https://image.slidesharecdn.com/kgf-chapter2-kinetics-221101172740-987afbc6/85/KGF-Chapter-2-Kinetics-pptx-3-320.jpg)
![• Consider the reaction A B
• There are two ways of measuring rate of reaction:
(1) the speed at which the reactants are vanished
(2) the speed at which the products are formed
• A general way of measuring the rate of the reaction is in terms of
change in concentration per unit time
Instantaneous rate =
∆[A]
∆t
limits to
d[A]
dt
Most Common Units… Rate = M/s
Where, Molarity (M) = moles/Liter](https://image.slidesharecdn.com/kgf-chapter2-kinetics-221101172740-987afbc6/85/KGF-Chapter-2-Kinetics-pptx-4-320.jpg)
![Reaction rate is the change in the concentration of a
reactant or a product with time (M/s).
A B
rate = -
∆[A]
∆ t
rate =
∆[B]
∆ t
∆[A] = change in concentration of A over
time period ∆ t
∆[B] = change in concentration of B over
time period ∆ t
Because [A] decreases with time, ∆[A] is negative.](https://image.slidesharecdn.com/kgf-chapter2-kinetics-221101172740-987afbc6/85/KGF-Chapter-2-Kinetics-pptx-5-320.jpg)
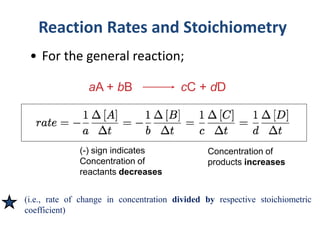
![Example;
For the reaction:
2N2O5(g) 4NO2(g) + O2(g)
we write the rate of the reaction as follows:
Reaction Rate = −
𝟏
𝟐
∆[N2O5]
∆t
=
𝟏
𝟒
∆[NO2]
∆t
=
∆[O2]
∆t](https://image.slidesharecdn.com/kgf-chapter2-kinetics-221101172740-987afbc6/85/KGF-Chapter-2-Kinetics-pptx-7-320.jpg)
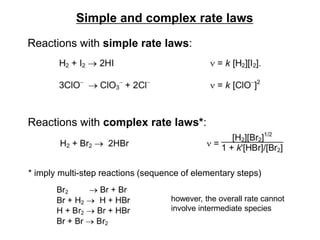
![Rate Law
• In general, rate of a reaction increases with the increase in
concentration of reactants since there are more collisions
occurring between reactants.
• The overall dependence of reaction rate on the reactant
concentration is given in the form of rate law.
• For the reaction; mA + nB pC + qD
Rate = k [A]m [B]n
[A] and [B] are reactant concentrations
The exponents m and n are reaction order (w.r.t. specific
reactant)
The constant k is the rate constant
The overall reaction order is the sum of the reaction orders
m + n](https://image.slidesharecdn.com/kgf-chapter2-kinetics-221101172740-987afbc6/85/KGF-Chapter-2-Kinetics-pptx-10-320.jpg)
![F2 (g) + 2ClO2 (g) 2FClO2 (g)
Rate = k [F2][ClO2]
• Rate law, rate constant and orders are determined
experimentally ONLY.
• The order of a reactant is NOT generally related to its
stoichiometric coefficient in a balanced chemical equation.
• For example;
1
Rate Law](https://image.slidesharecdn.com/kgf-chapter2-kinetics-221101172740-987afbc6/85/KGF-Chapter-2-Kinetics-pptx-11-320.jpg)
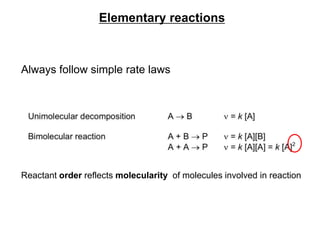
![Determination of the order of an equation
For example ;
Exp.No. Concentration of [A]
mol/l or (M)
Concentration of
[B] mol/l or (M)
Rate M/s
1 0.1 0.1 0.002
2 0.2 0.1 0.004
3 0.2 0.2 0.016
0.002 = k[0.1]n[0.1]m and 0.004 = k[0.2]n[0.1]m
Dividing, 0.004/0.002 = k[0.2]n[0.1]m / k[0.1]n[0.1]m
Therefore n = 1
Similarly, 0.016 = k [0.2]n[0.2]m and 0.004 = k [0.2]n[0.1]m
Dividing and simplifying , 4 = 2m ; 22 = 2m ; m = 2
The order of the equation is m + n = 2+1 = 3](https://image.slidesharecdn.com/kgf-chapter2-kinetics-221101172740-987afbc6/85/KGF-Chapter-2-Kinetics-pptx-14-320.jpg)