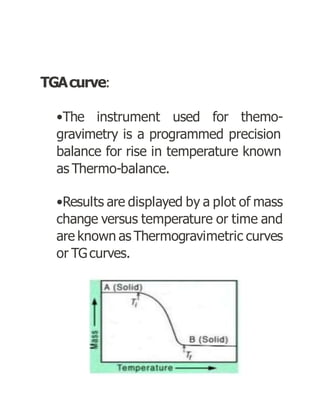
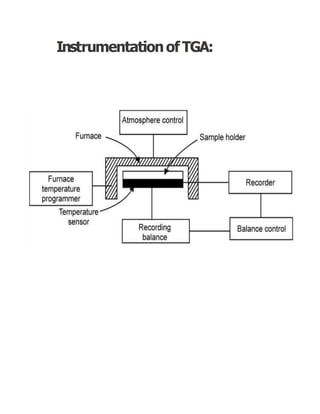
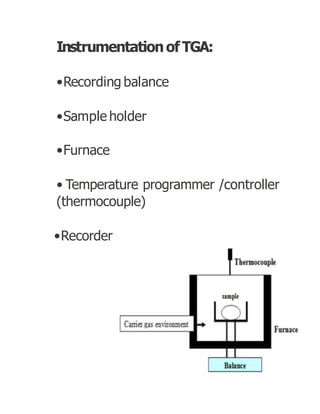
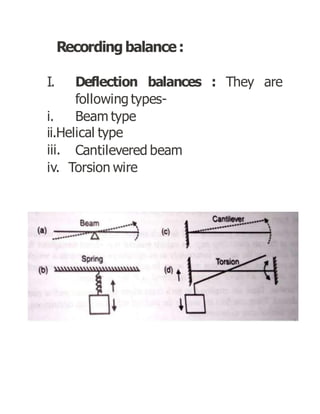
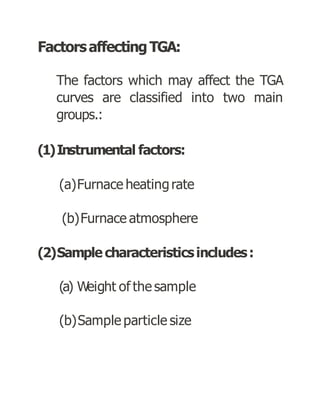
Thermogravimetric analysis (TGA) measures the mass of a sample as the temperature changes. There are three main types: isothermal, quasistatic, and dynamic. TGA provides information about physical and chemical phenomena like phase transitions and decomposition reactions. The sample is heated and weight changes are measured and plotted in a thermogravimetric curve. TGA is used to study material properties, composition, and stability in applications like pharmaceutical analysis and catalyst studies.