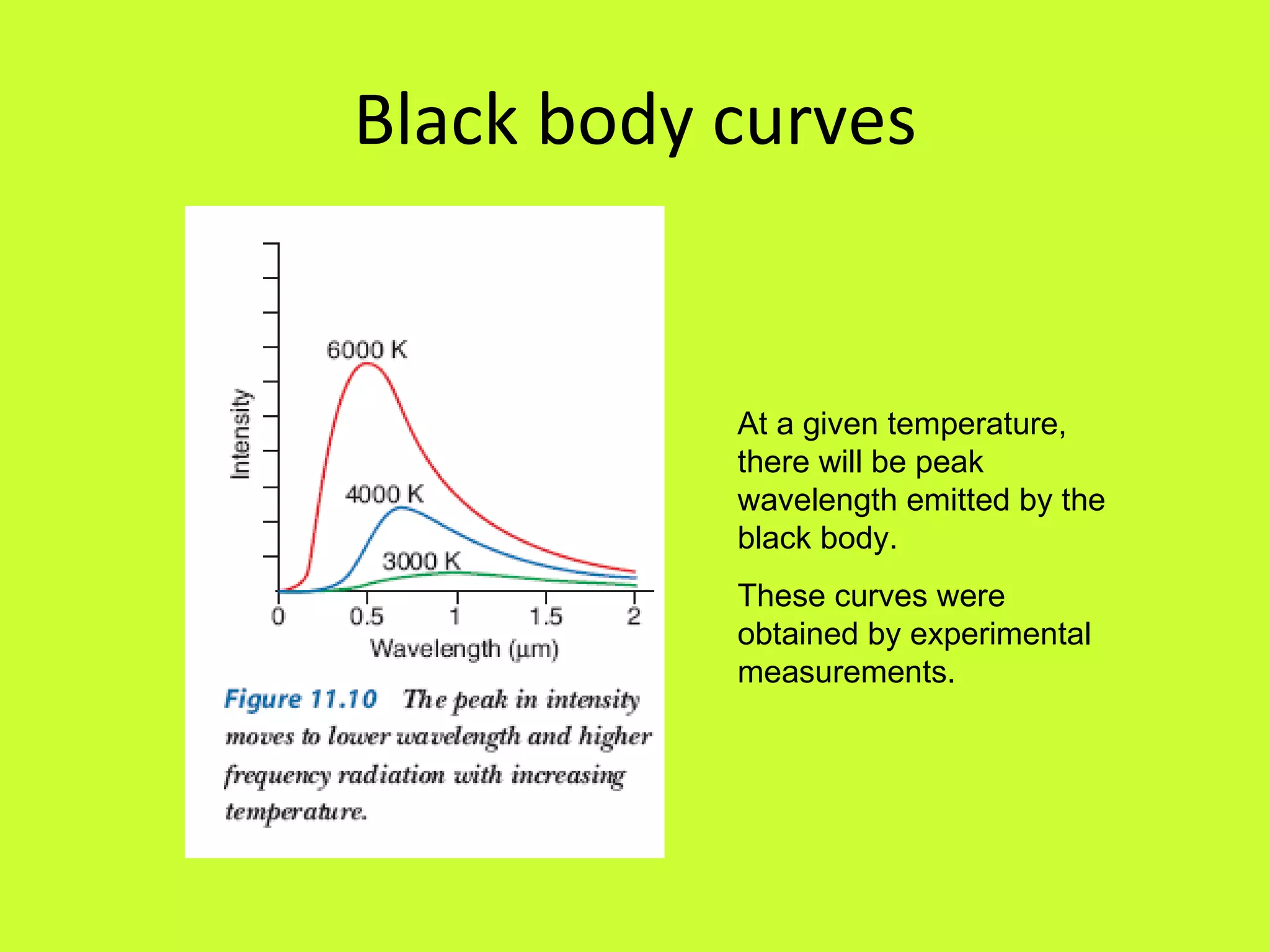
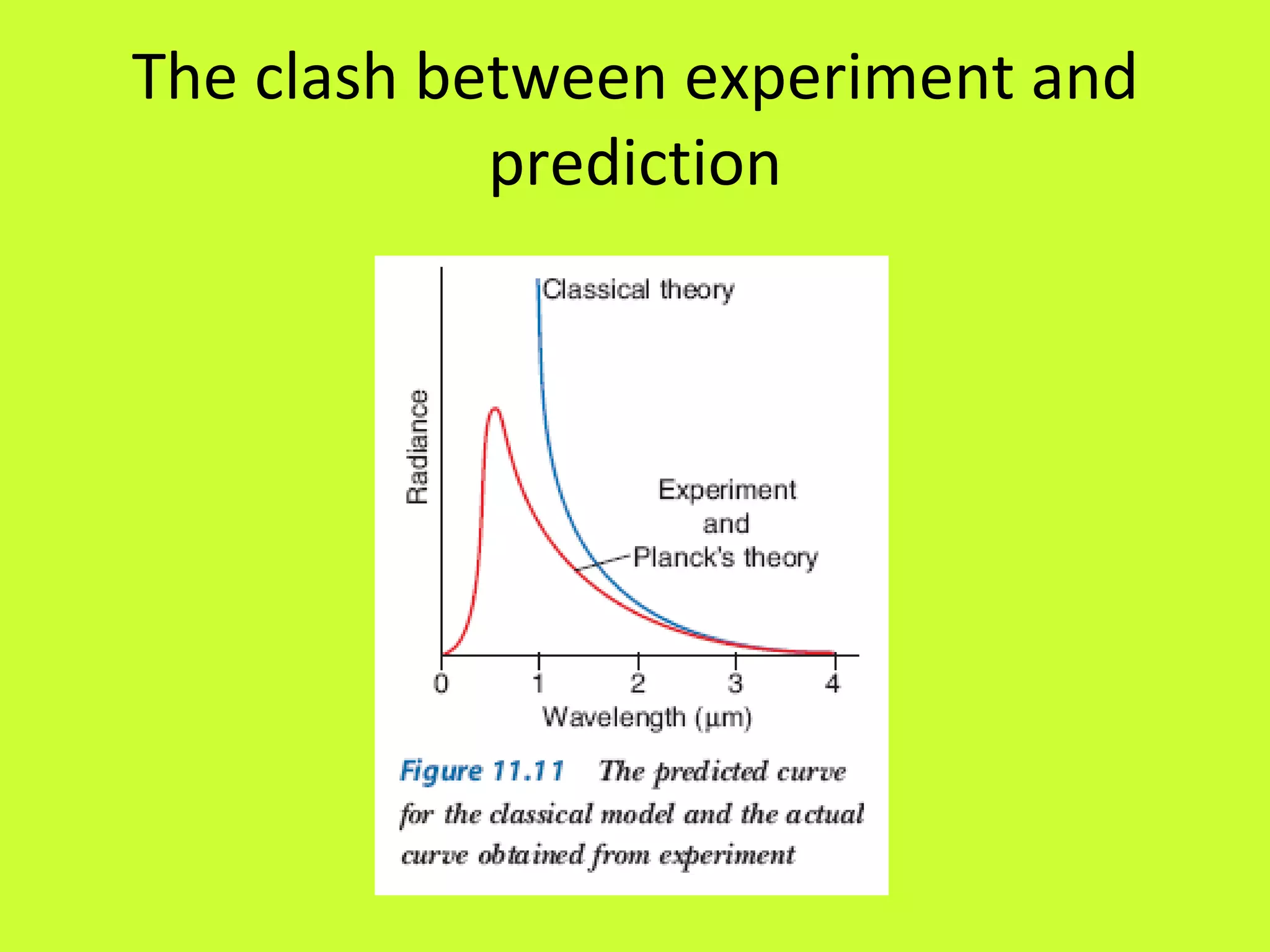
1. Quantum theory proposes that energy is transferred in discrete quanta or packets rather than continuously, as first suggested by Max Planck in 1900 to explain blackbody radiation curves.
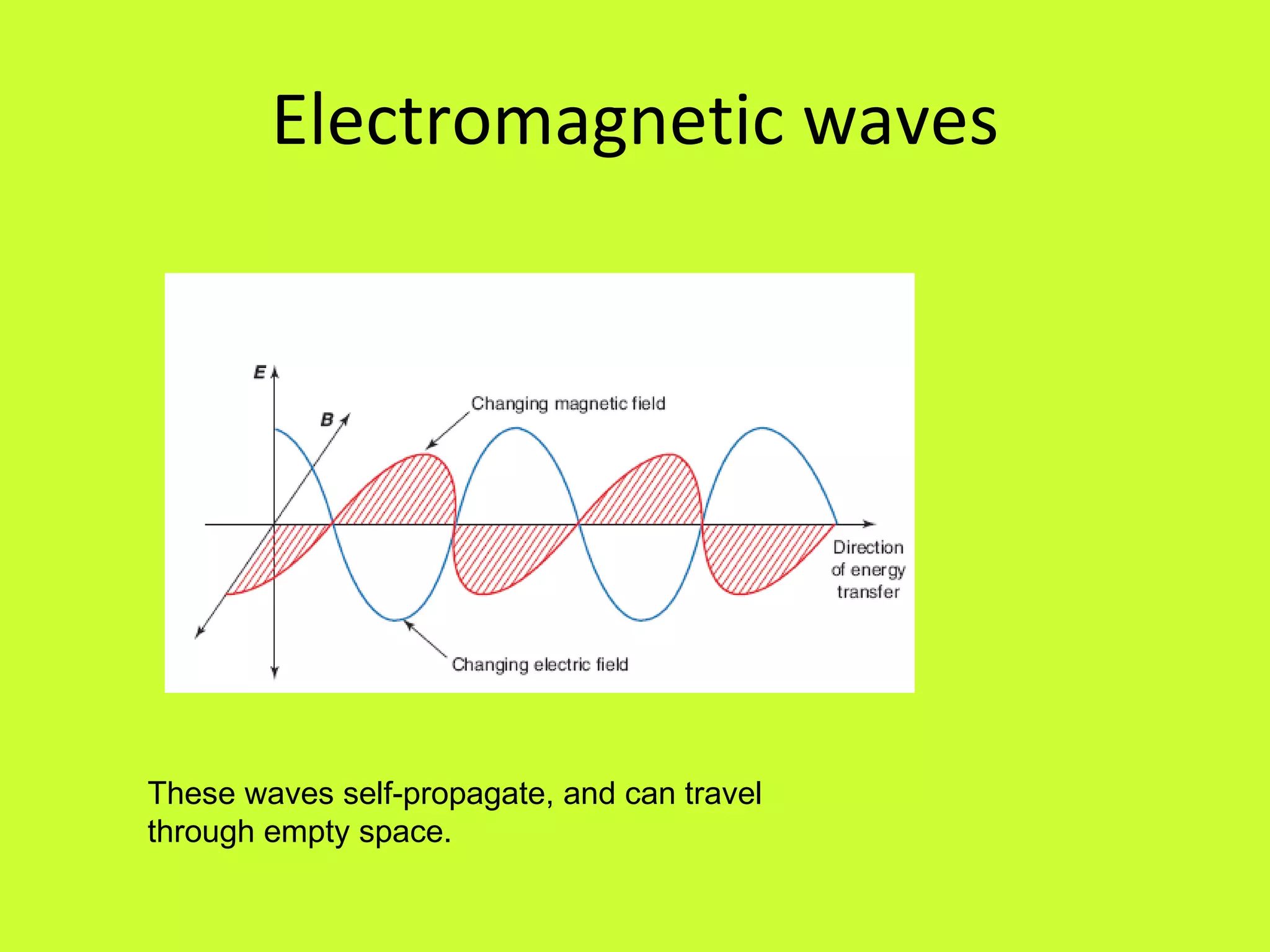
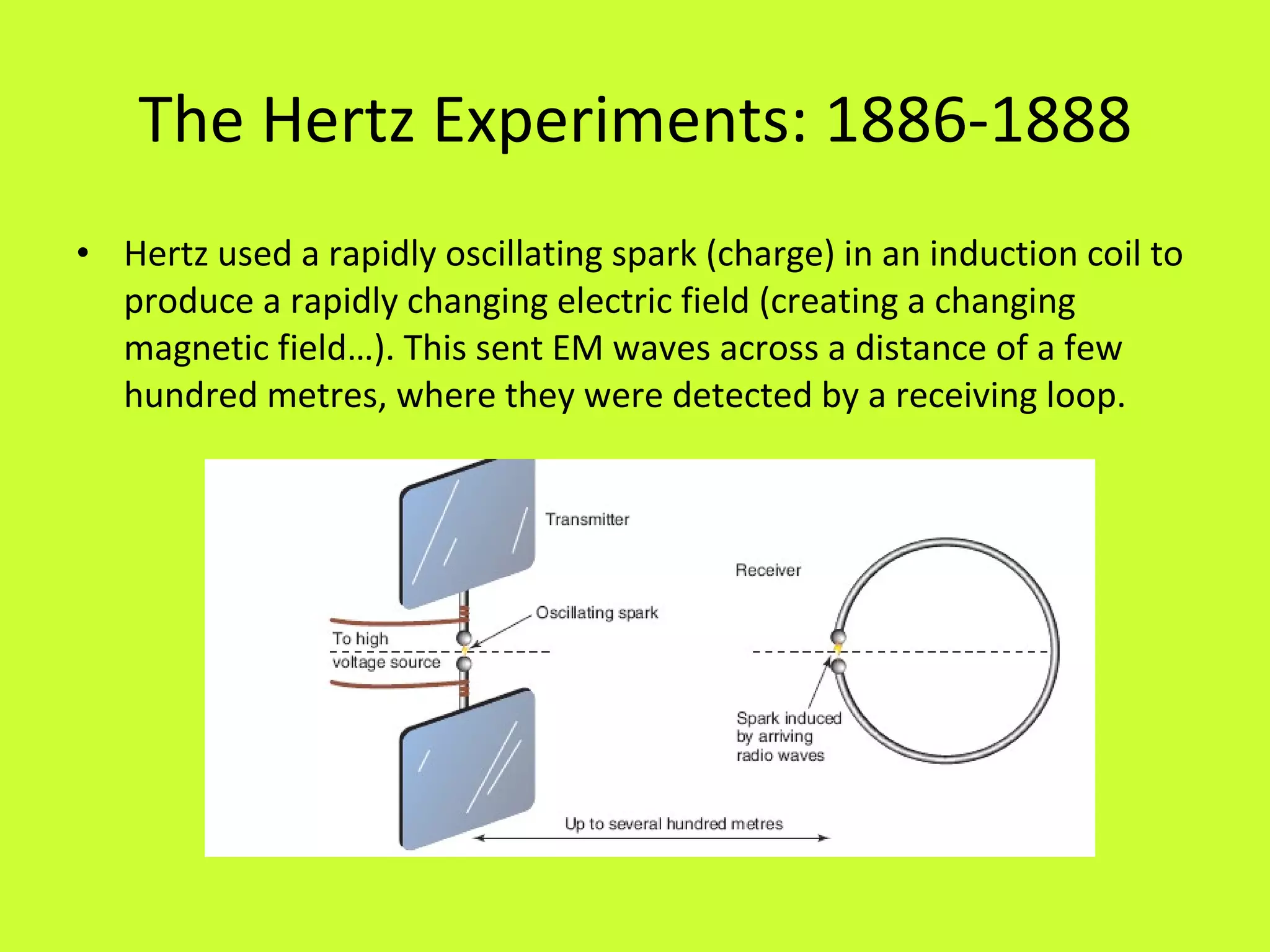
2. Heinrich Hertz experimentally demonstrated electromagnetic radiation in the late 1880s by generating and detecting radio waves, verifying James Clerk Maxwell's theory of electromagnetism.
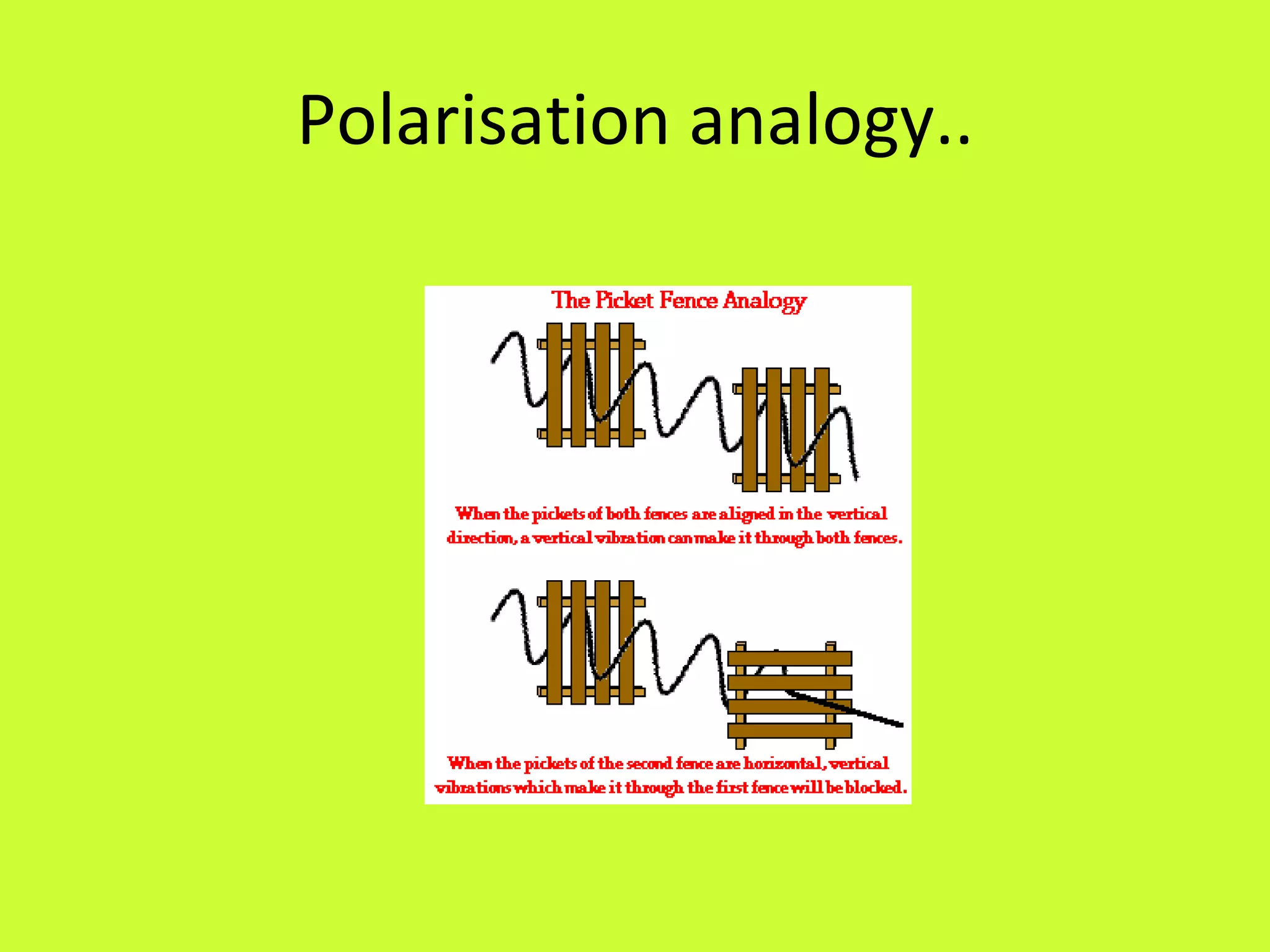
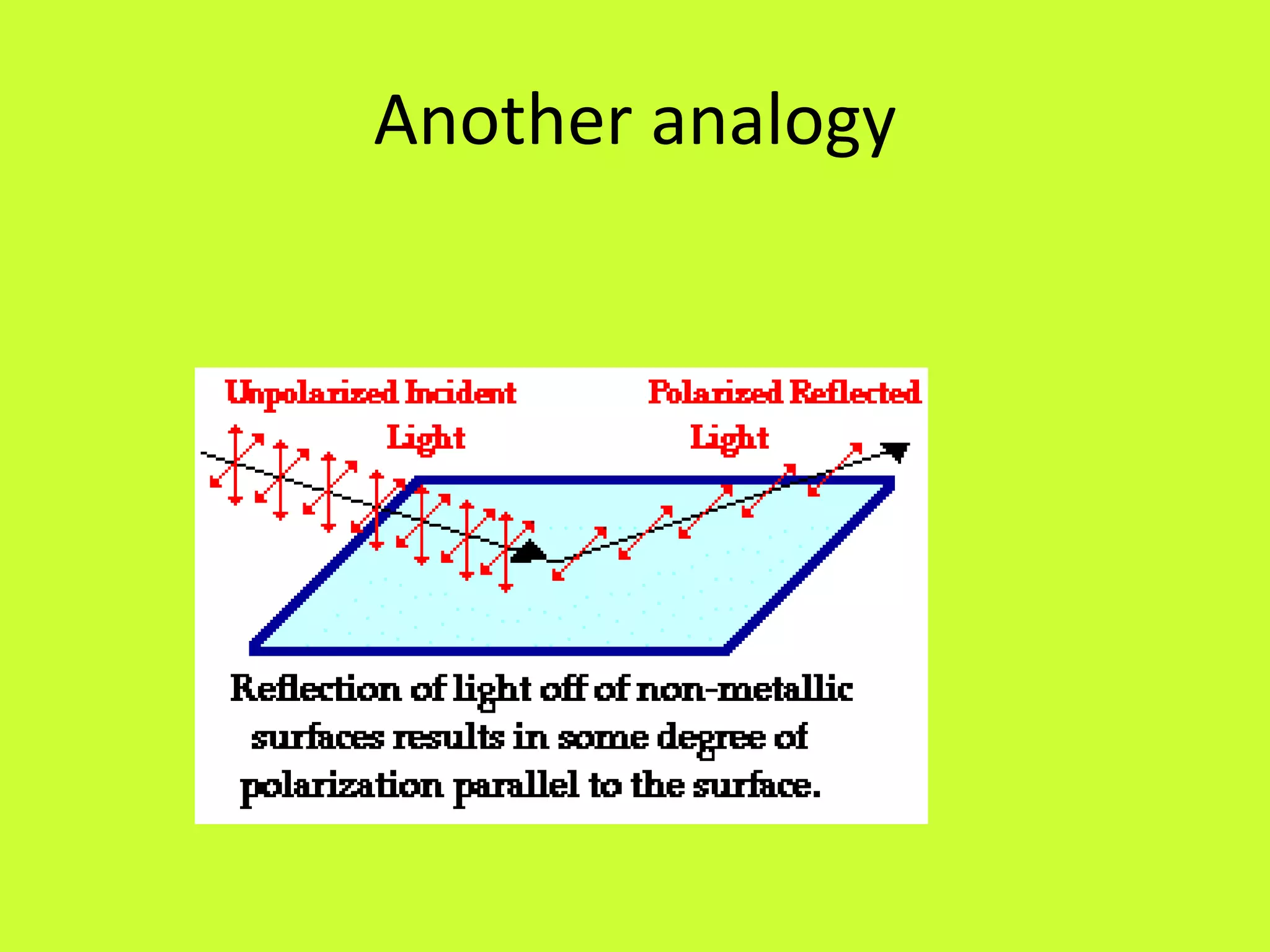
3. Hertz made important discoveries about the properties of electromagnetic waves, including their ability to be reflected, refracted, and polarized, but did not realize the significance of his work.