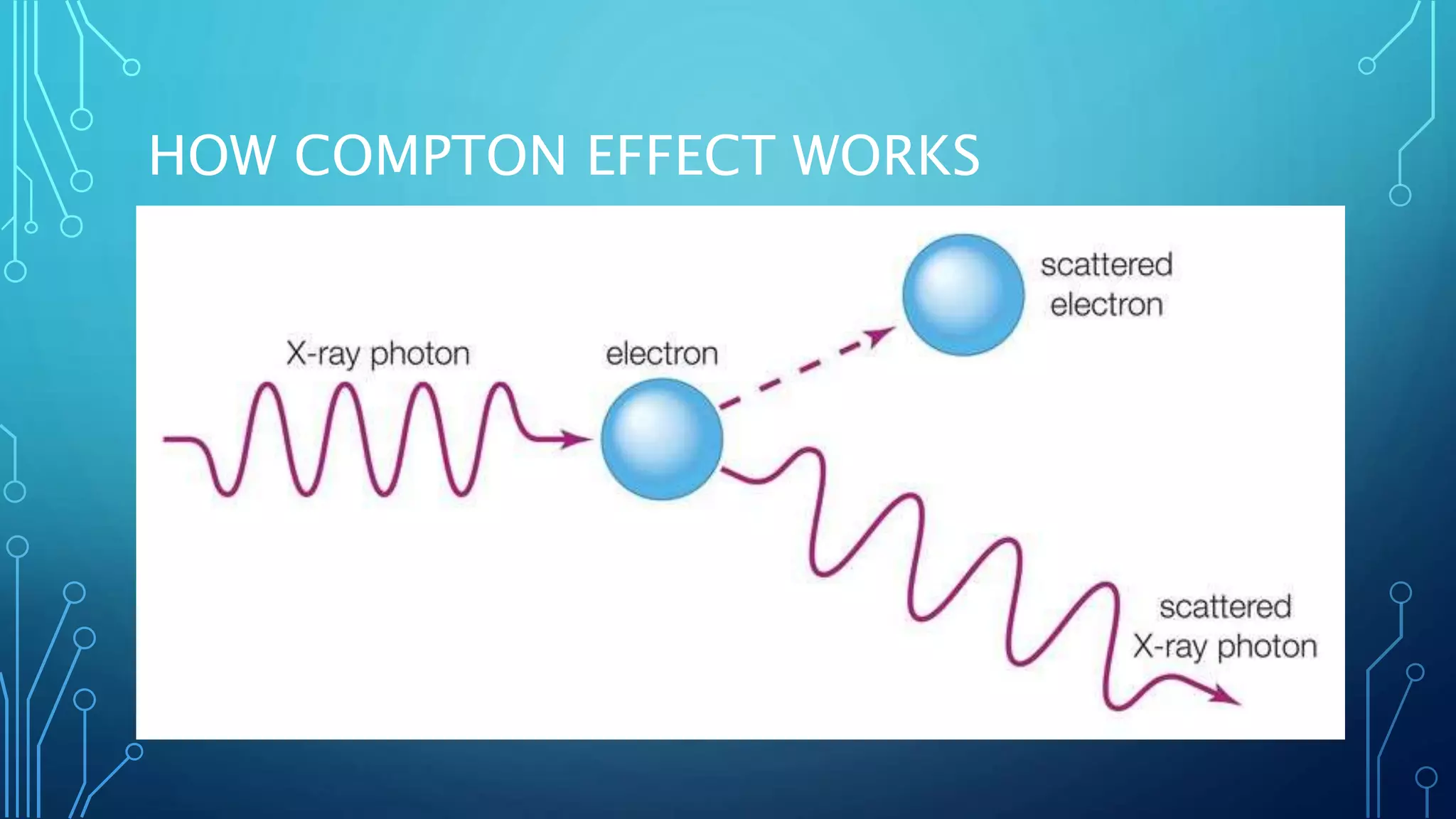
The Compton effect occurs when a high-energy photon collides with an electron, causing the photon to lose some energy and increase in wavelength. Arthur Holly Compton discovered this effect in 1923 through experiments bombarding a graphite target with x-rays and measuring the wavelength of scattered radiation. The effect showed that light behaves as both a particle and wave and is important in fields like radiation therapy and gamma spectroscopy. It is explained by the transfer of momentum and energy between the photon and electron during collision.