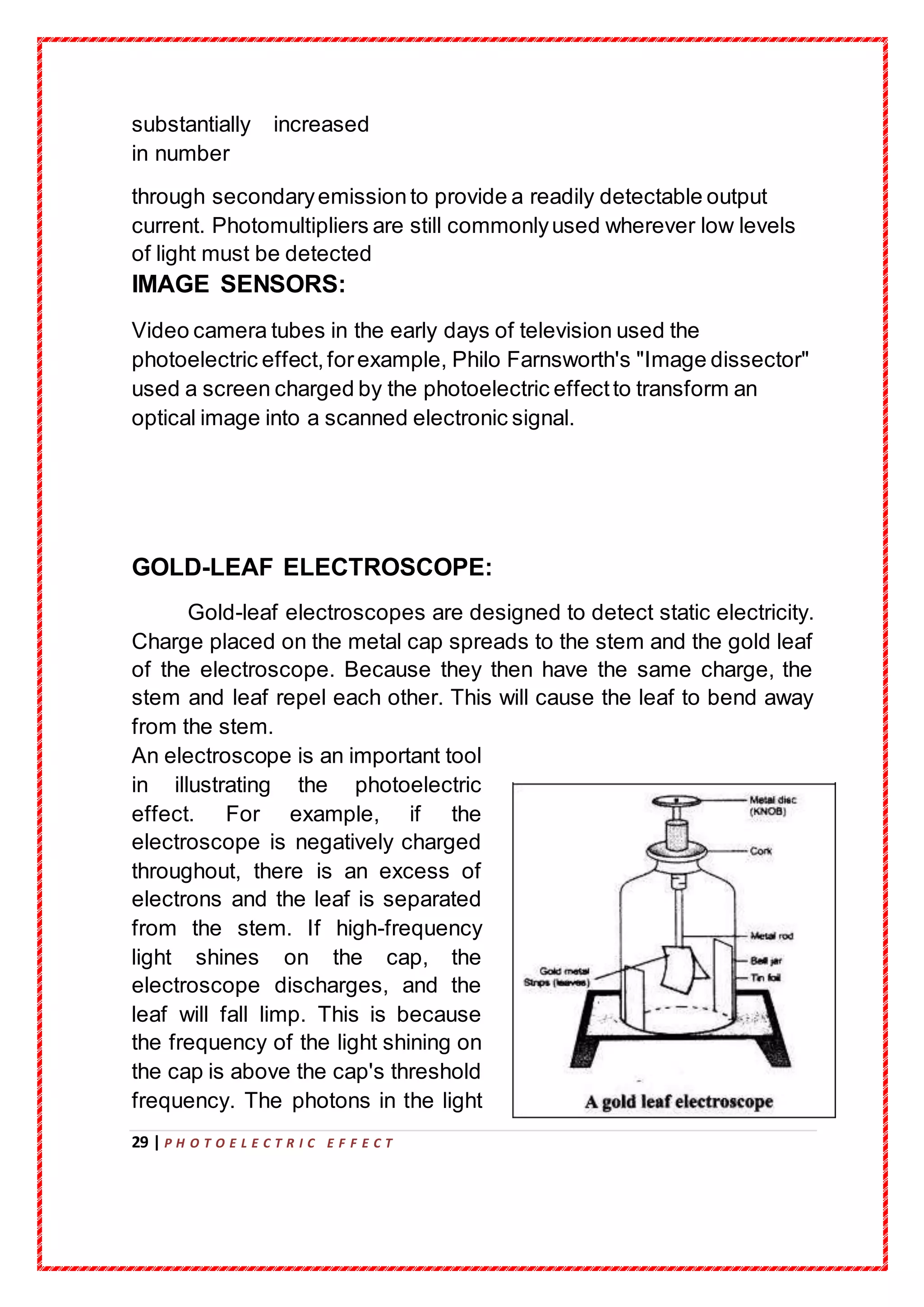
The document details a project submission on the photoelectric effect as part of the AISSCE requirements at RMK Senior Secondary School for the academic year 2019-20. It covers the historical context, experimental observations, and the theoretical framework surrounding the photoelectric effect, including contributions from prominent physicists such as Hertz, Millikan, and Einstein. The study emphasizes the importance of photons and quantum mechanics in explaining the phenomenon of electron emission due to light exposure.