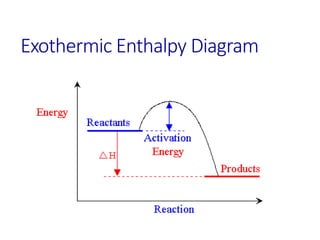
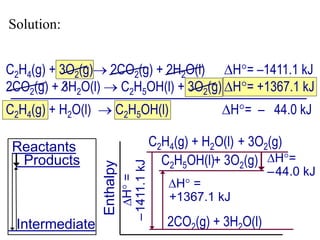
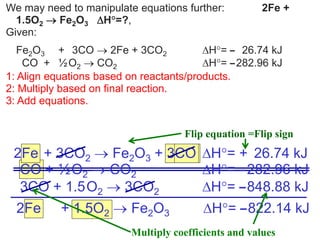
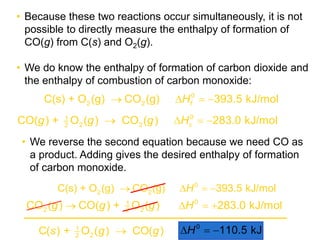
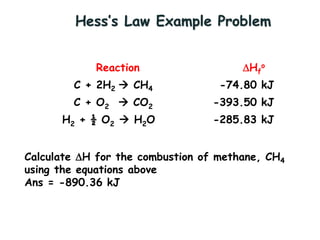
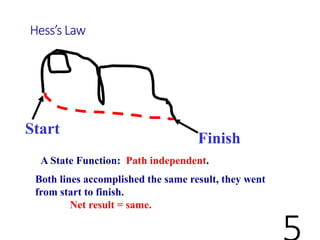
Hess's law states that the total enthalpy change for a reaction is equal to the sum of the enthalpy changes of the steps that make up that reaction. This allows one to calculate the enthalpy of a reaction from standard enthalpy of formation values without directly measuring the enthalpy change experimentally. Enthalpy diagrams can be used to visually represent how individual reaction steps add together based on Hess's law.










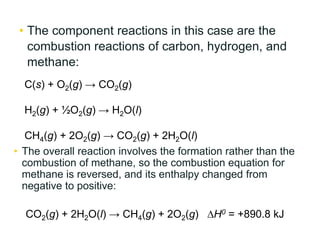

![• Using Hess’s law, any enthalpy of reaction may be
calculated using enthalpies of formation for all the
substances in the reaction of interest, without
knowing anything else about how the reaction occurs.
• Mathematically, the overall equation for enthalpy
change will be in the form of the following equation:
∆H0 = sum of [( of products) × (mol of products)]
– sum of [( of reactants) × (mol of reactants)]
fH0
fH0
](https://image.slidesharecdn.com/lesson3hesslaw-170906085950/85/Lesson-3-hess-law-15-320.jpg)