Embed presentation
Download to read offline

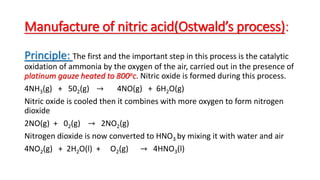
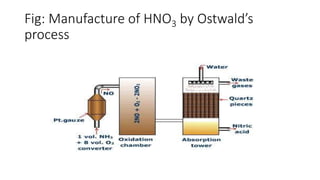


Nitric acid is manufactured through Ostwald's process, which involves the catalytic oxidation of ammonia by air over a platinum catalyst heated to 800°C, forming nitric oxide. The nitric oxide is cooled and oxidized to nitrogen dioxide, which is then absorbed in water in an absorption tower to produce nitric acid solution. The dilute nitric acid is further concentrated by distillation under reduced pressure to obtain approximately 98% nitric acid.




