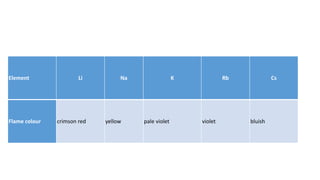
- Alkali metals have low ionization energies and readily lose their outer electron to form cations with a +1 oxidation state. They are soft, reactive metals that form ionic compounds.
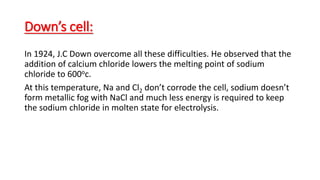
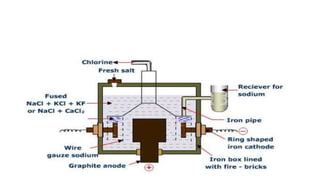
- Sodium is the second alkali metal and is found abundantly in nature as the mineral sodium chloride. It is extracted commercially via the Downs process, which involves electrolysis of molten sodium chloride at lower temperatures using calcium chloride. This allows pure sodium to be produced at the cathode and chlorine gas to be collected at the anode.

![Periodic discussion and general characteristics of
alkali metals:
Electronic configurations
All the alkali metals have one electron in their outermost 's' orbitals
preceded by the noble gas configuration. Thus, the general
configuration of alkali metals may be written as [Noble gas] ns1 where
'n' represents the valence shell. The electronic configurations of alkali
metals are:
The electronic configurations of alkali metals are as follows:](https://image.slidesharecdn.com/sodium-180114120834/85/Sodium-2-320.jpg)
![Element Symbol Atomic No.
Electronic
configuration
Lithium Li 3 [He]2s1
Sodium Na 11 [Ne]3s1
Potassium K 19 [Ar]4s1
Rubidium Rb 37 [Kr]5s1
Cesium Cs 55 [Xe]6s1
Francium Fr 87 [Rn]7s1](https://image.slidesharecdn.com/sodium-180114120834/85/Sodium-3-320.jpg)