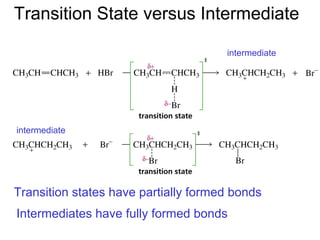
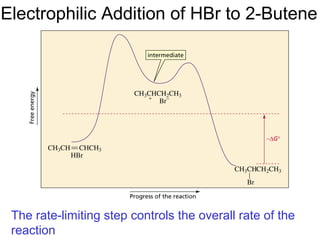
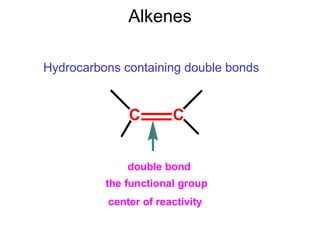
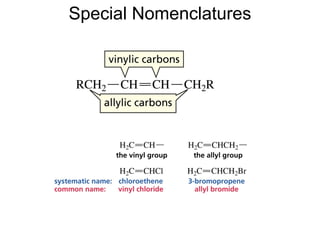
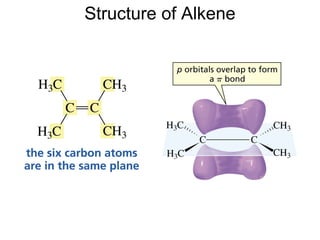
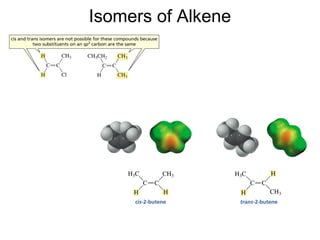
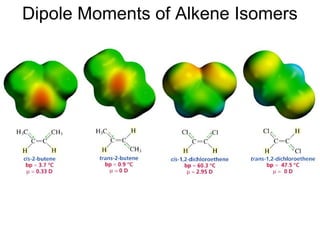
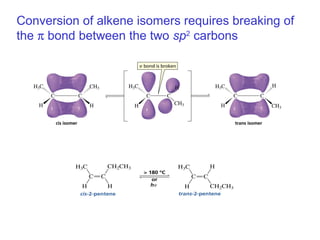
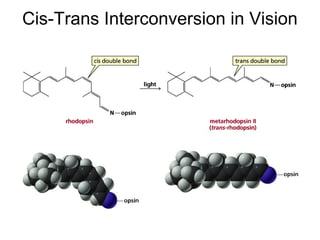
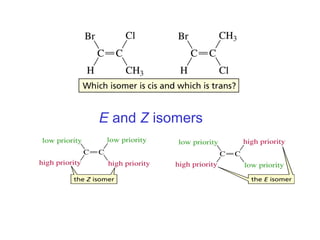
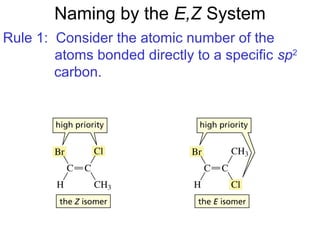
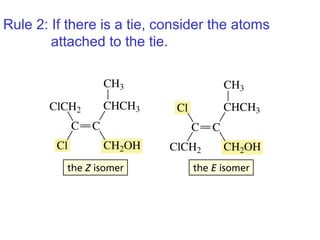
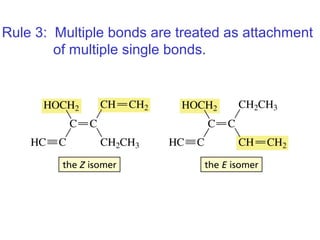
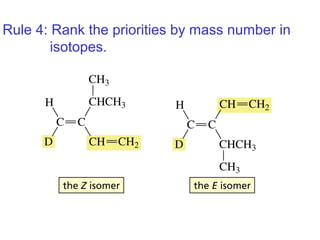
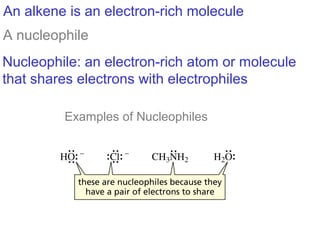
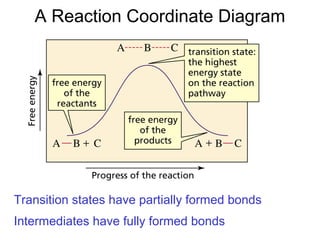
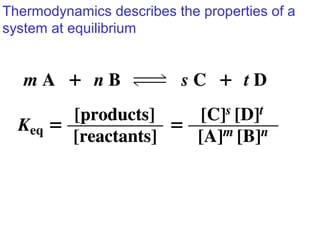
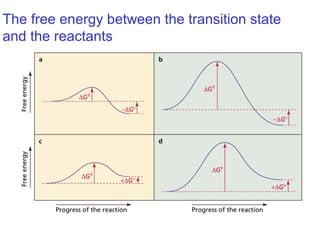
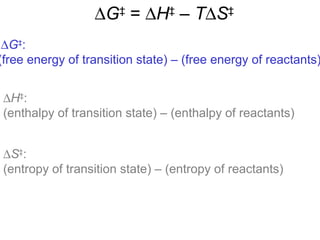
This document provides an overview of organic chemistry concepts related to alkenes including their structures, nomenclature, isomerism, reactivity, and reaction mechanisms. Key points covered include the molecular formula and naming conventions of alkenes, cis-trans isomerism, nucleophilic and electrophilic addition reactions, and the thermodynamic and kinetic parameters that govern reaction rates such as activation energy, rate constants, and reaction order.
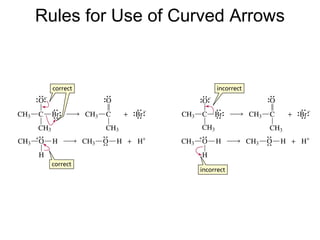
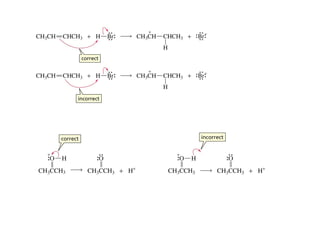
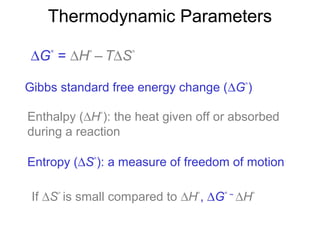
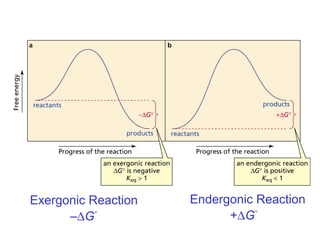
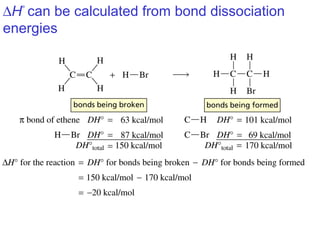
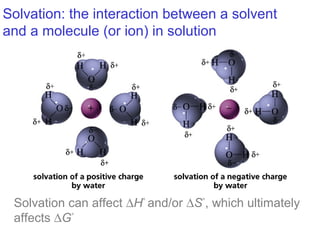
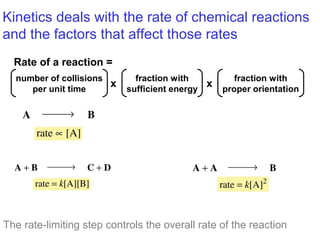
![Rates and Rate Constants
First-order reaction
A B
rate = k[A]
Second-order
reaction
A + B C + D
rate = k[A][B]](https://image.slidesharecdn.com/ch03mr-150226143814-conversion-gate02/85/Chapter-3-Alkenes-Structures-Nomenclature-and-an-Introduction-to-Reactivity-Thermodynamics-and-Kinetics-34-320.jpg)
![The Arrhenius Equation
k = Ae
–Ea/RT
Ea = ∆H‡
+ RT
Rate Constants and the Equilibrium
Constant
A B
k1
k–1
Keq = k1/k–1 = [B]/[A]](https://image.slidesharecdn.com/ch03mr-150226143814-conversion-gate02/85/Chapter-3-Alkenes-Structures-Nomenclature-and-an-Introduction-to-Reactivity-Thermodynamics-and-Kinetics-35-320.jpg)