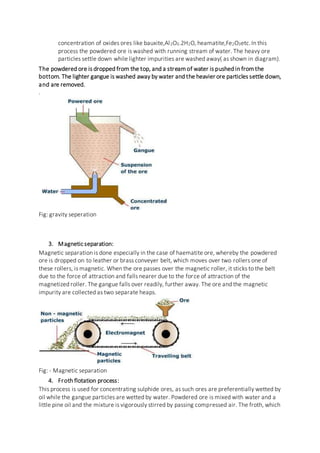
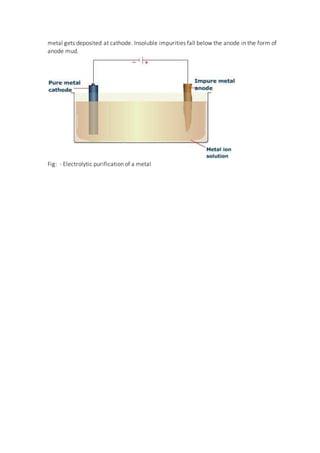
The document discusses the various processes involved in metallurgy, which is the extraction of metals from ores and their purification. There are three main types of metallurgical processes - pyrometallurgy which uses high temperatures, hydrometallurgy which uses aqueous solutions, and electrometallurgy which uses electrolysis. Common steps in metal extraction include mining the ore, crushing and grinding it, concentrating the ore to remove impurities, roasting or calcining it, reducing the metal oxides to the pure metal, and finally refining the metal through processes like liquation, distillation, or electrolysis.