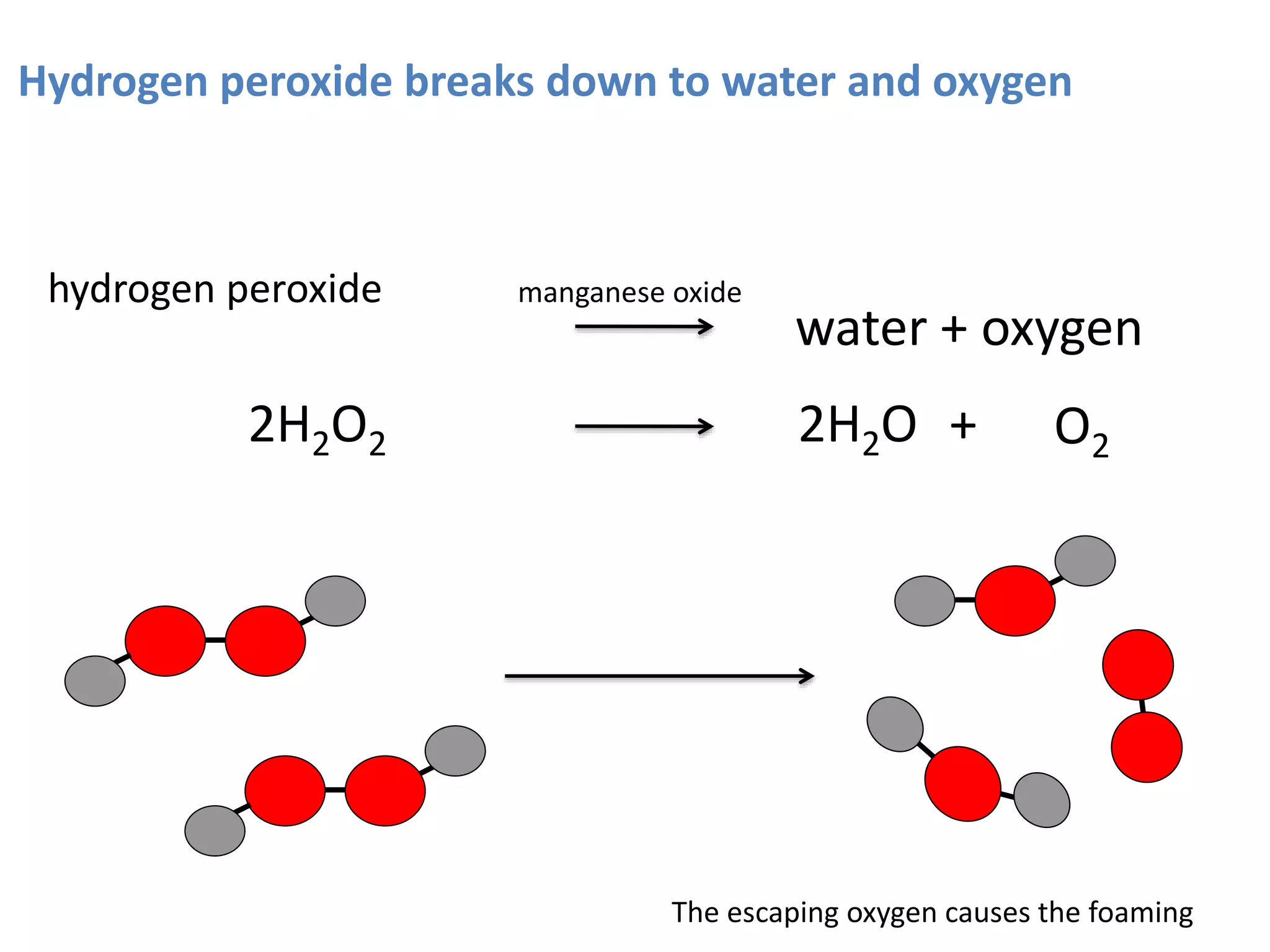
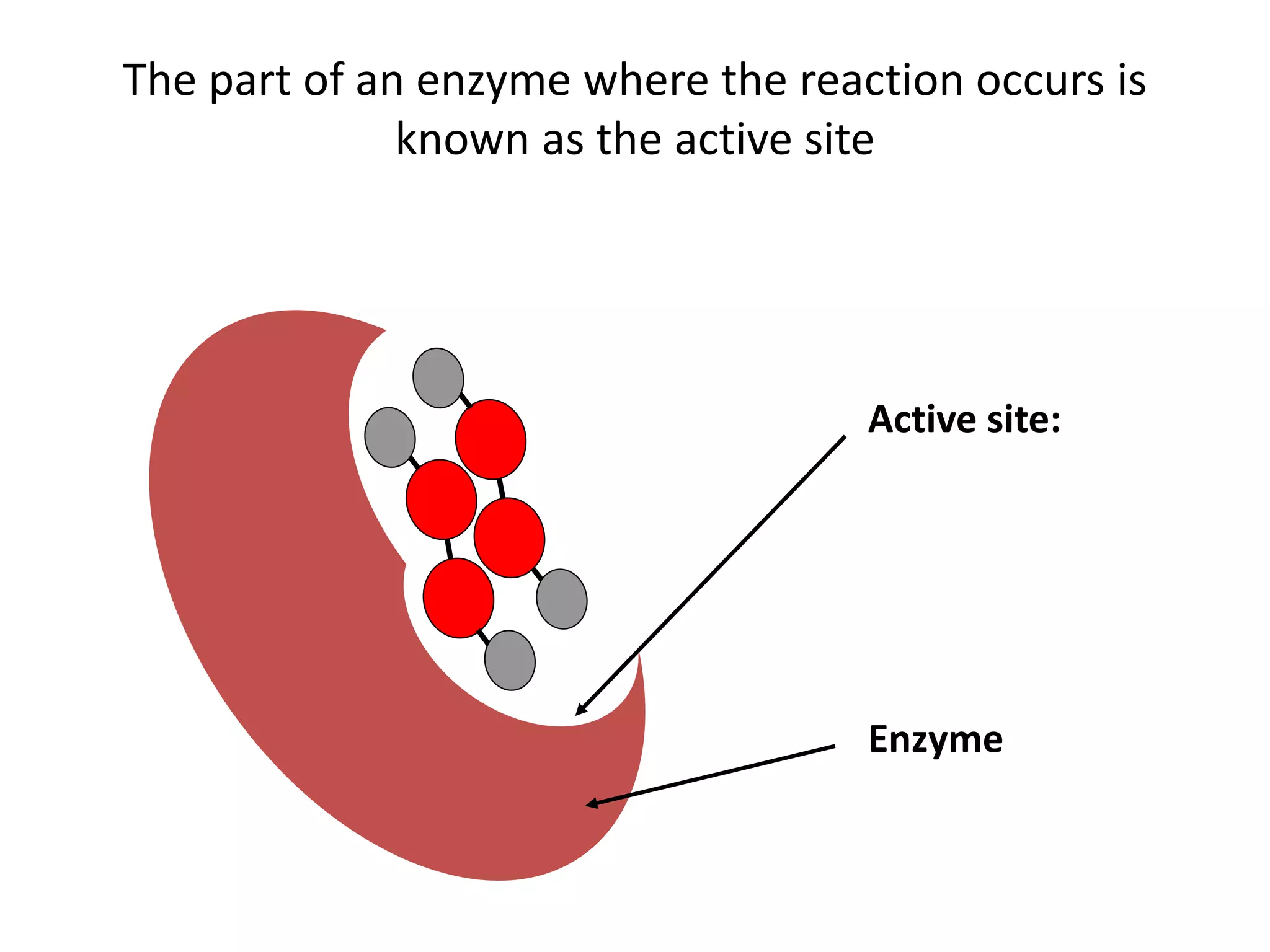
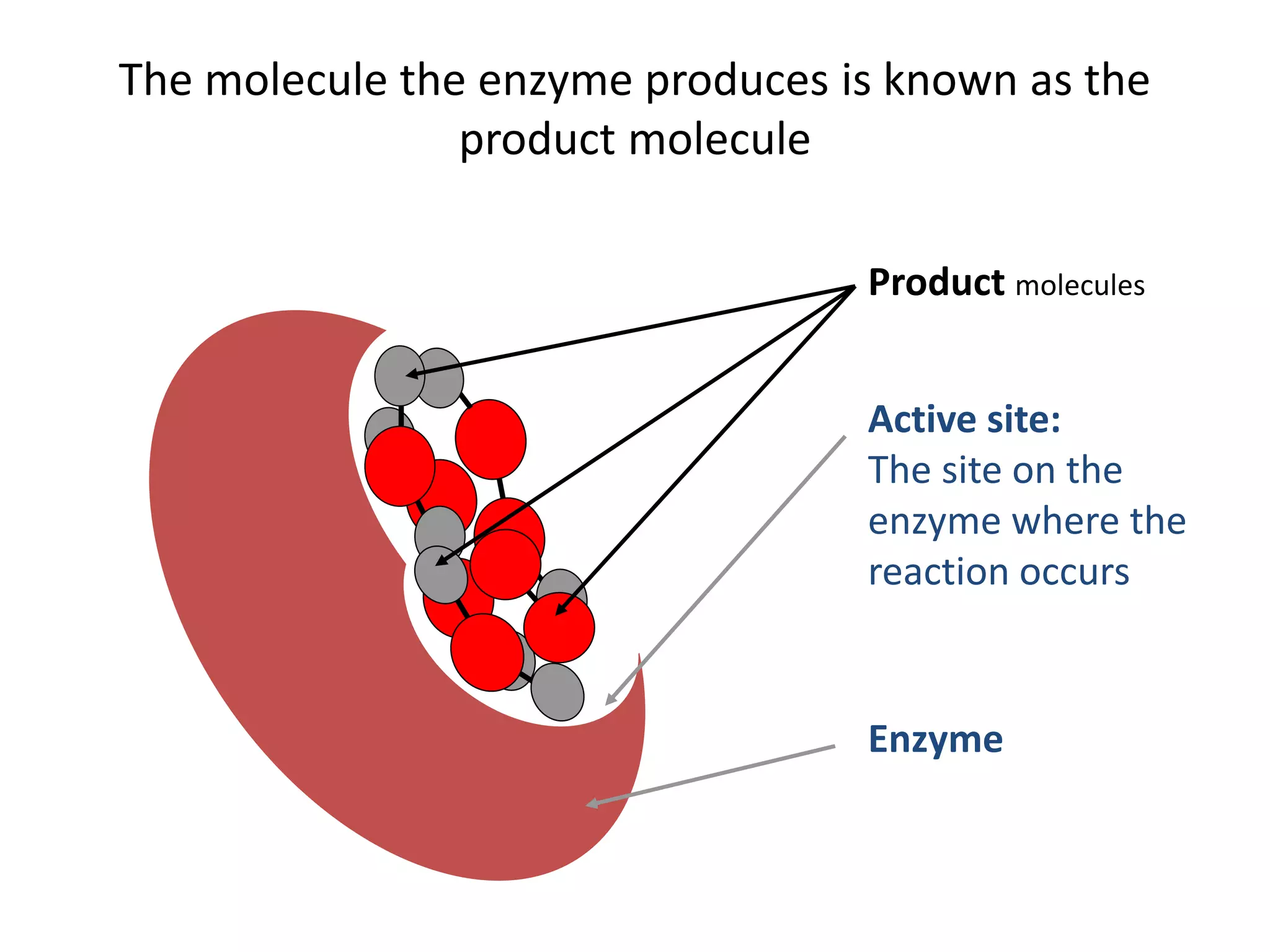
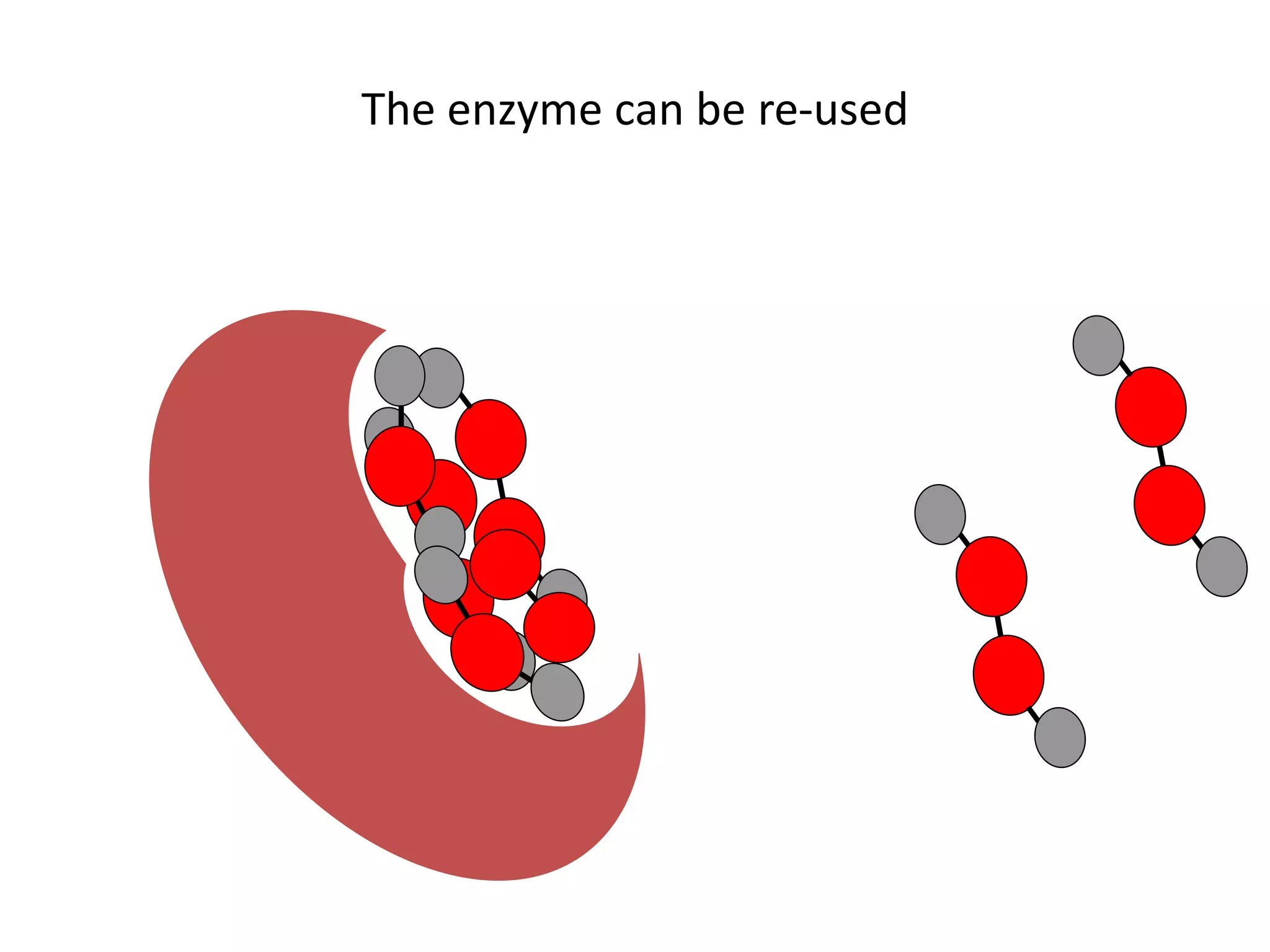
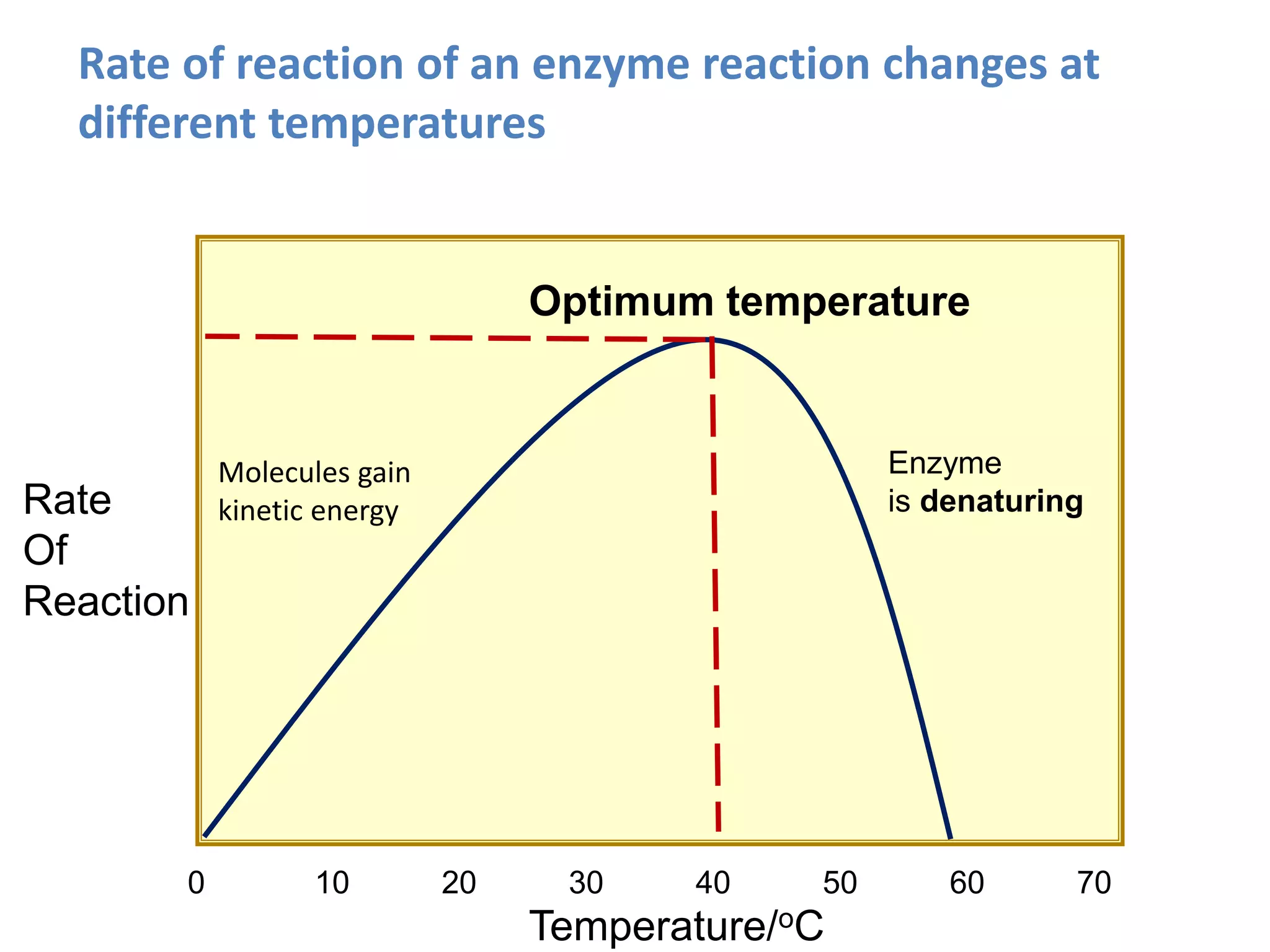
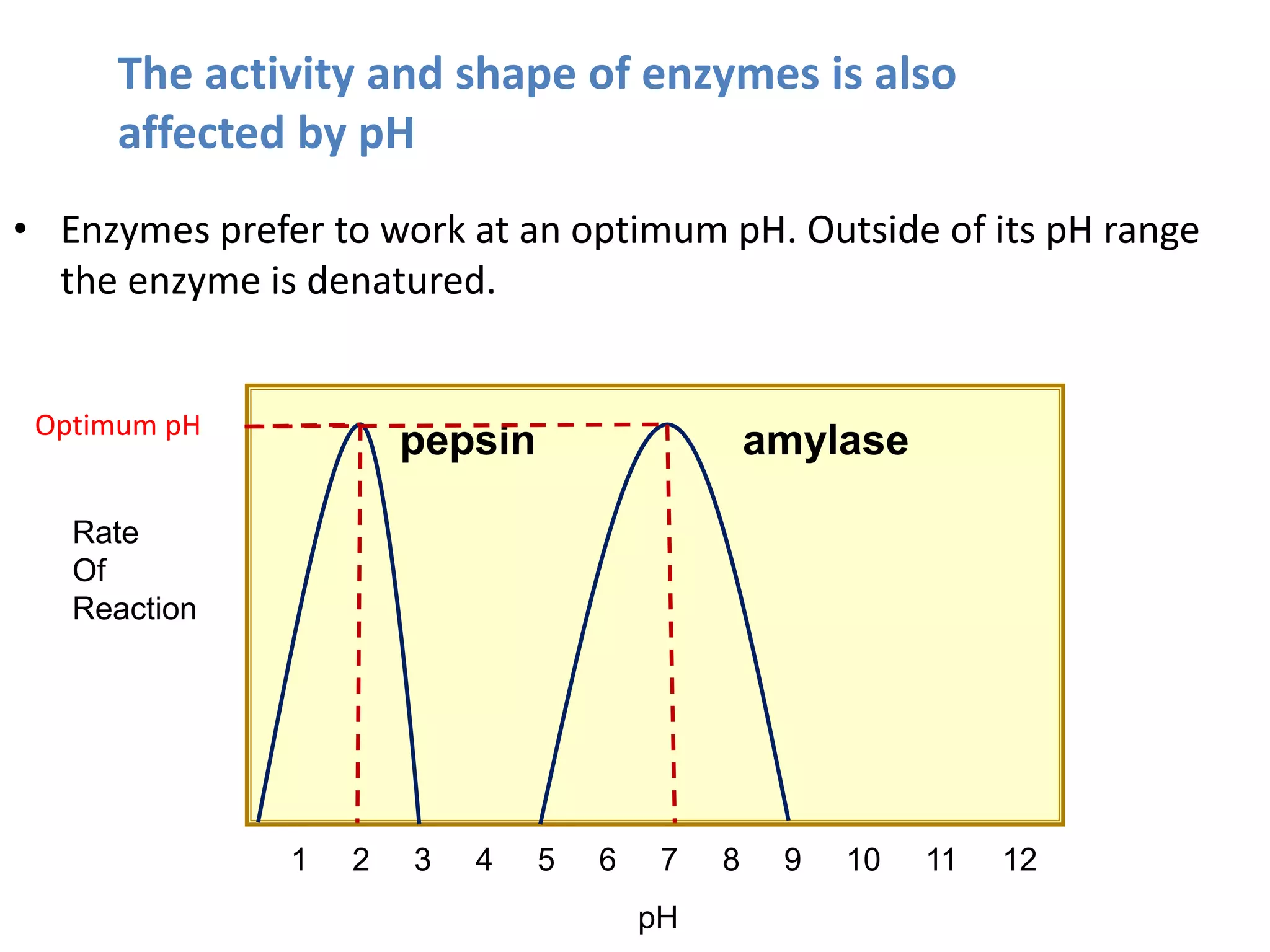
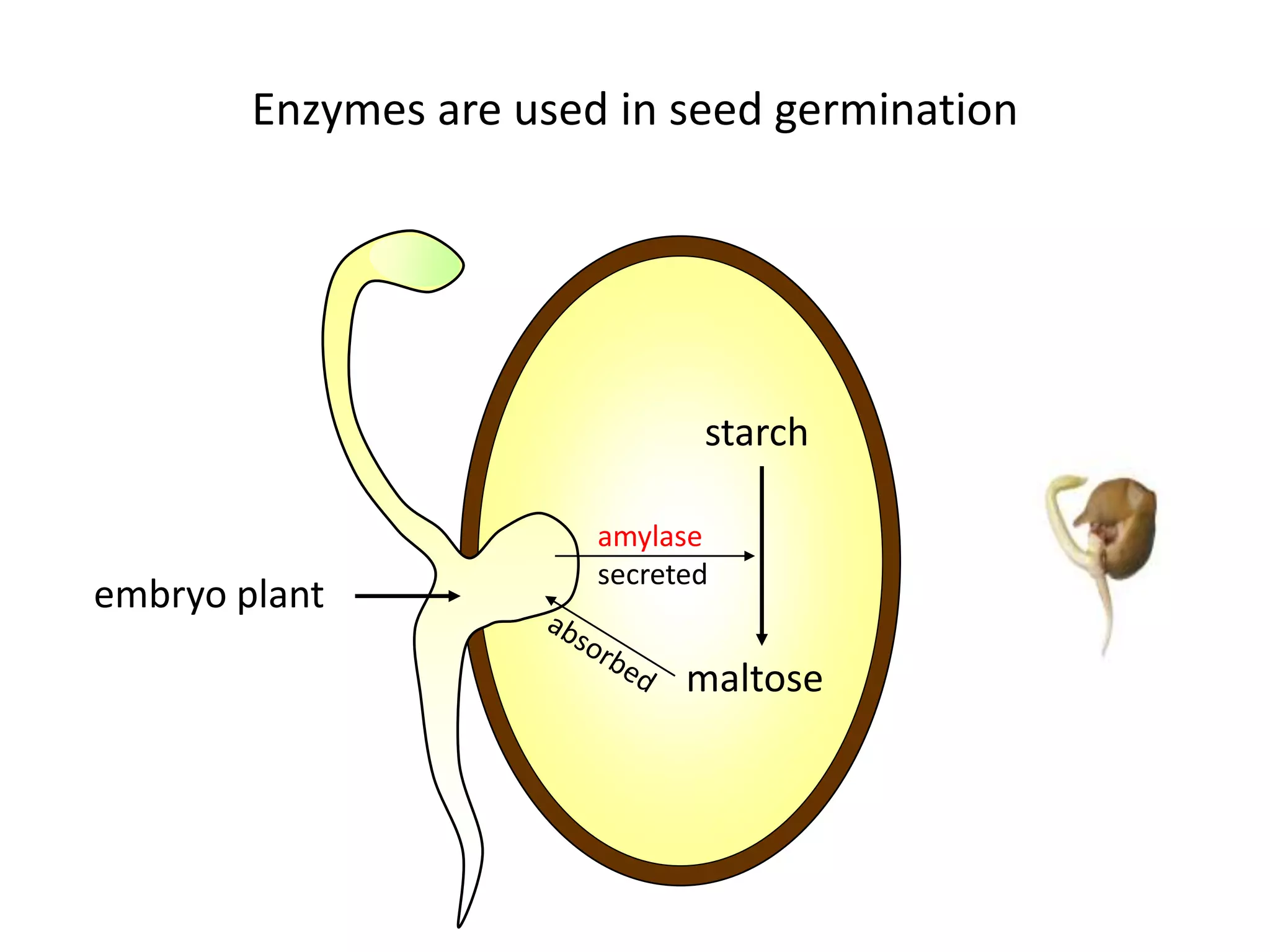
Enzymes are protein catalysts that speed up chemical reactions without being consumed. They have an active site that binds to a specific substrate molecule. As the substrate binds to the active site, it undergoes a reaction to form product molecules. Enzyme activity is optimized at certain temperatures and pH levels, but high heat or extreme pH can cause the enzyme to denature, or lose its shape. Enzymes play important roles in biology and industry, from seed germination to food processing and stain removal.