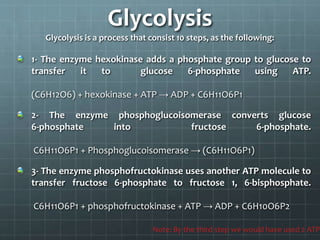
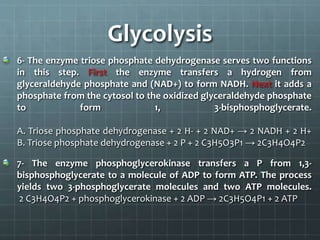
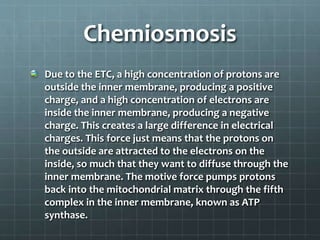
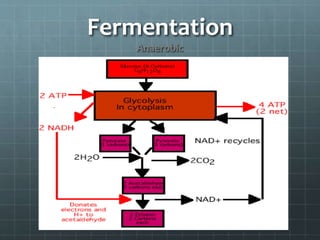
Cellular respiration has three main stages: glycolysis, the citric acid cycle, and oxidative phosphorylation. Glycolysis breaks down glucose into pyruvate and produces a small amount of ATP. The citric acid cycle further breaks down pyruvate and produces more ATP and electron carriers. During oxidative phosphorylation, electrons are passed through an electron transport chain which pumps protons across a membrane, establishing a proton gradient. ATP synthase uses this gradient to produce most of the cell's ATP. Fermentation can also produce a small amount of ATP without oxygen. Cellular respiration and fermentation are important catabolic processes that provide energy to fuel anabolism and power cellular functions. Diseases can arise if there are defects in acetyl-Co