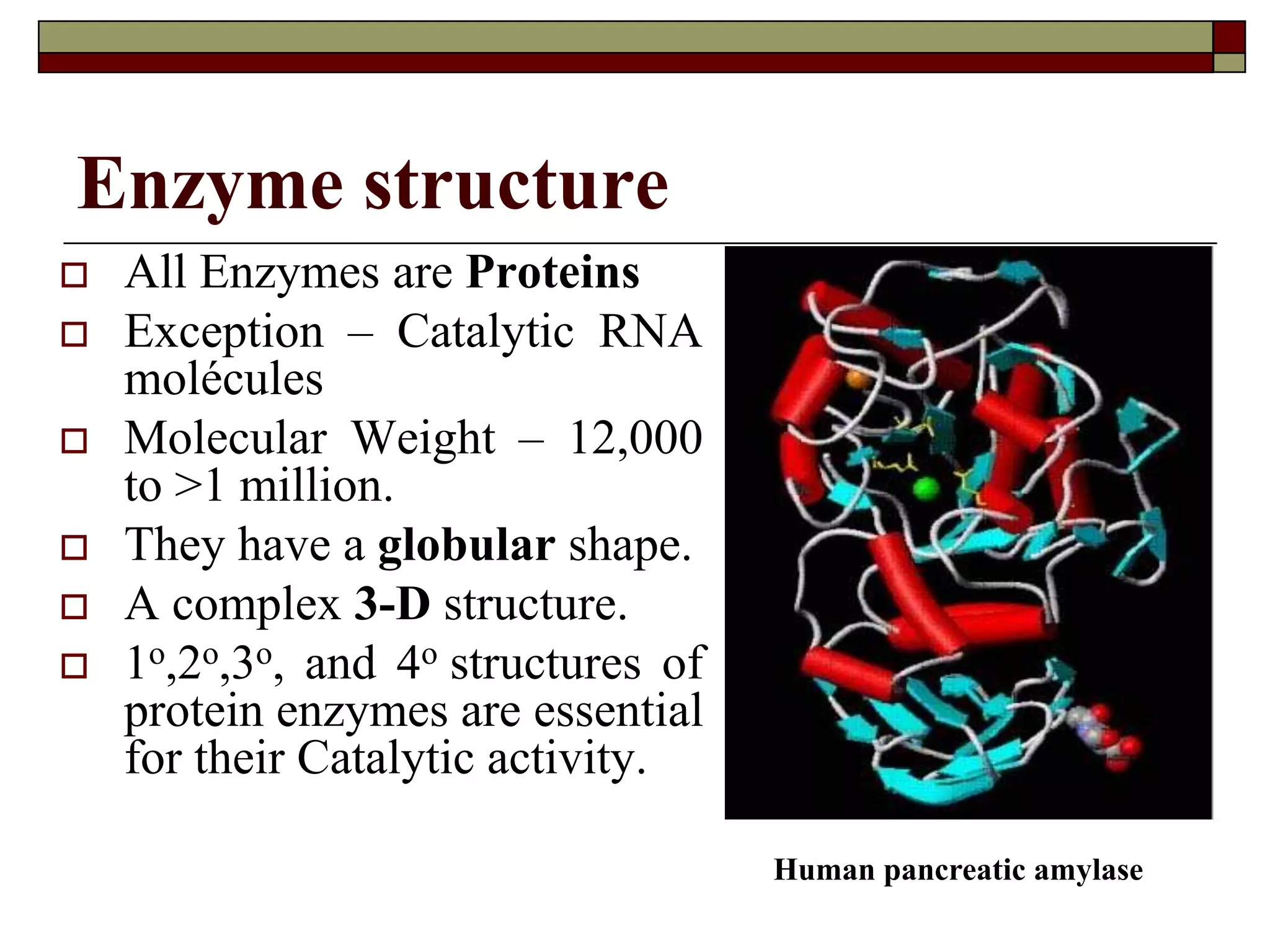
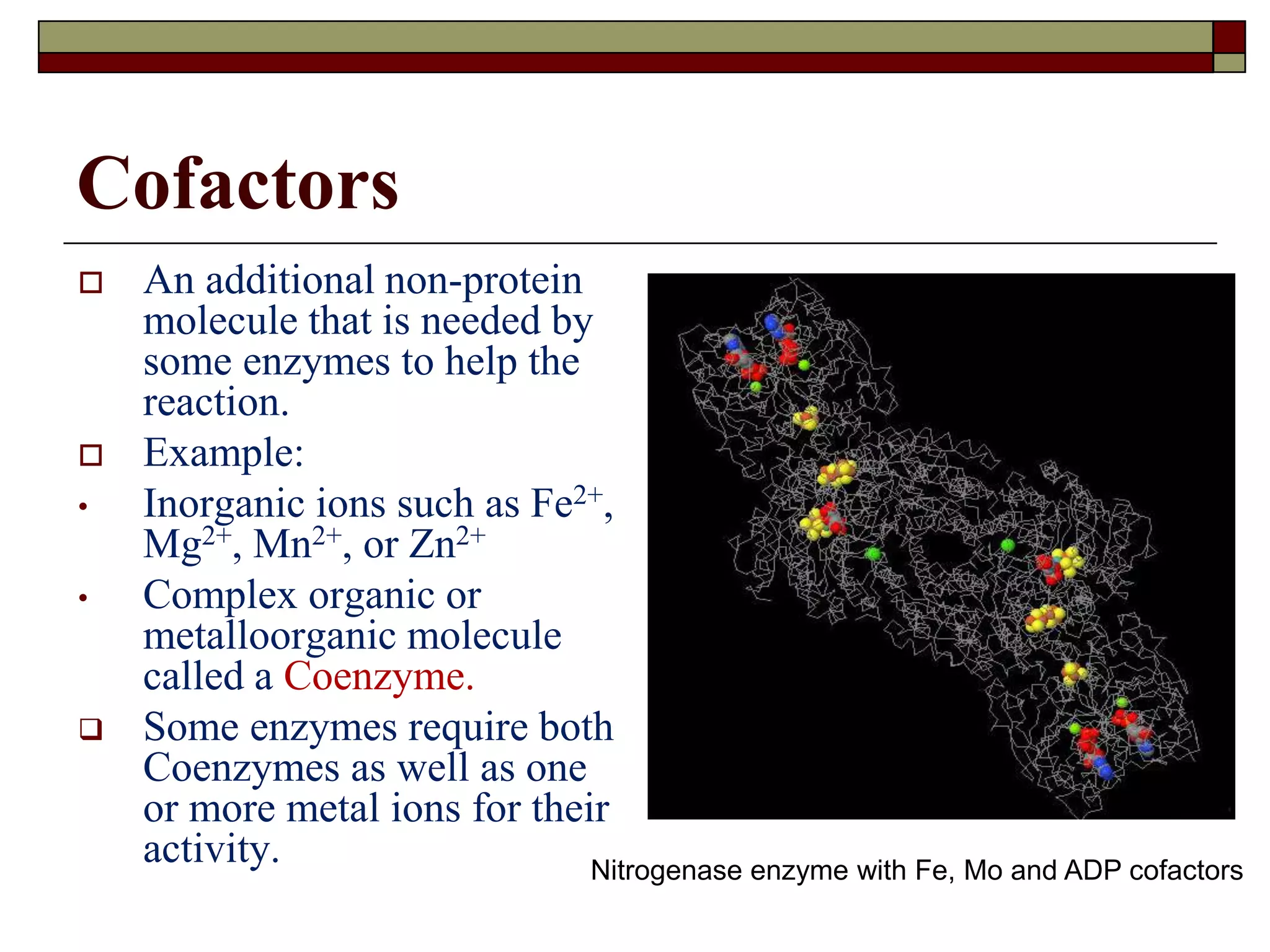
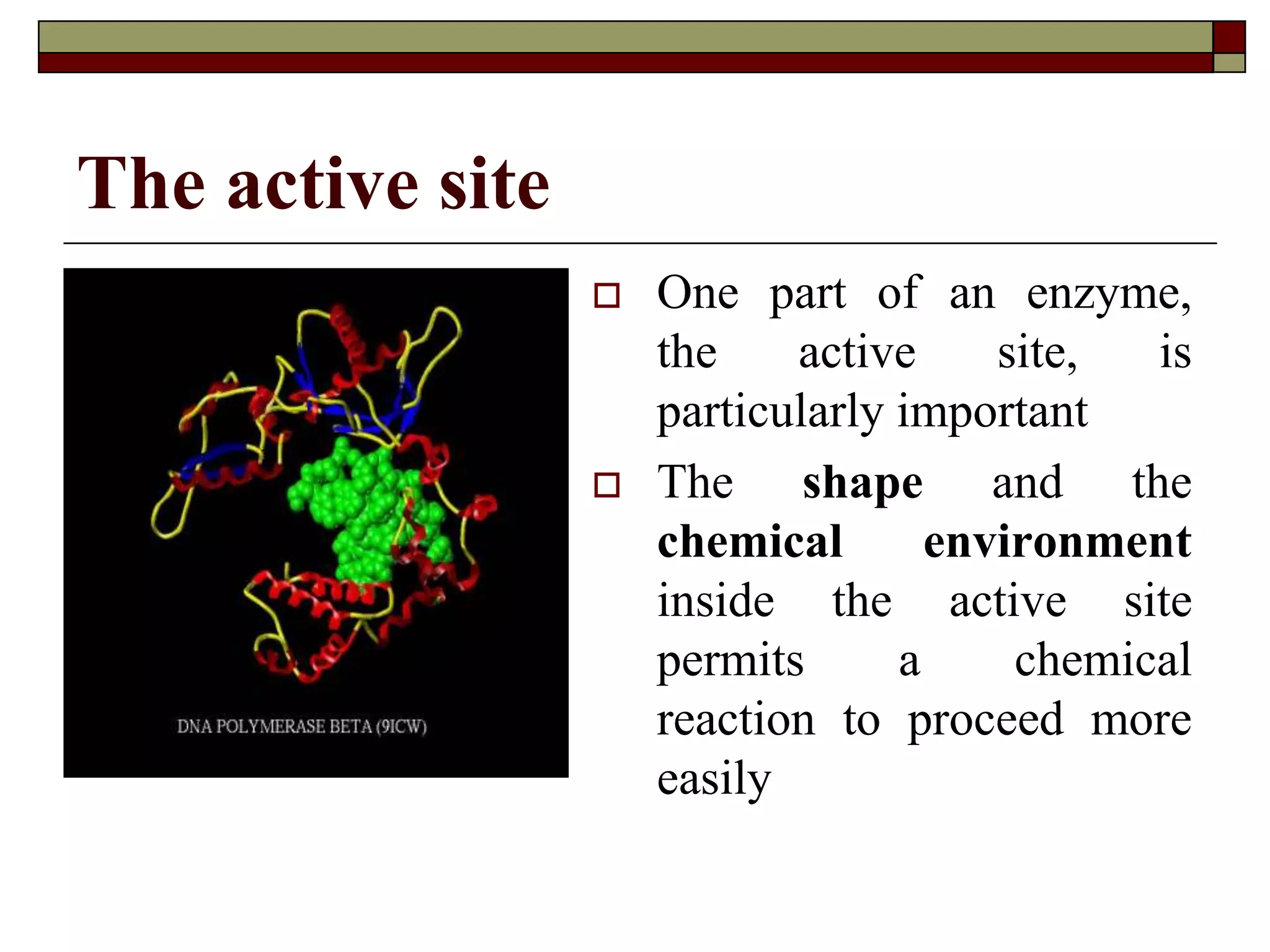
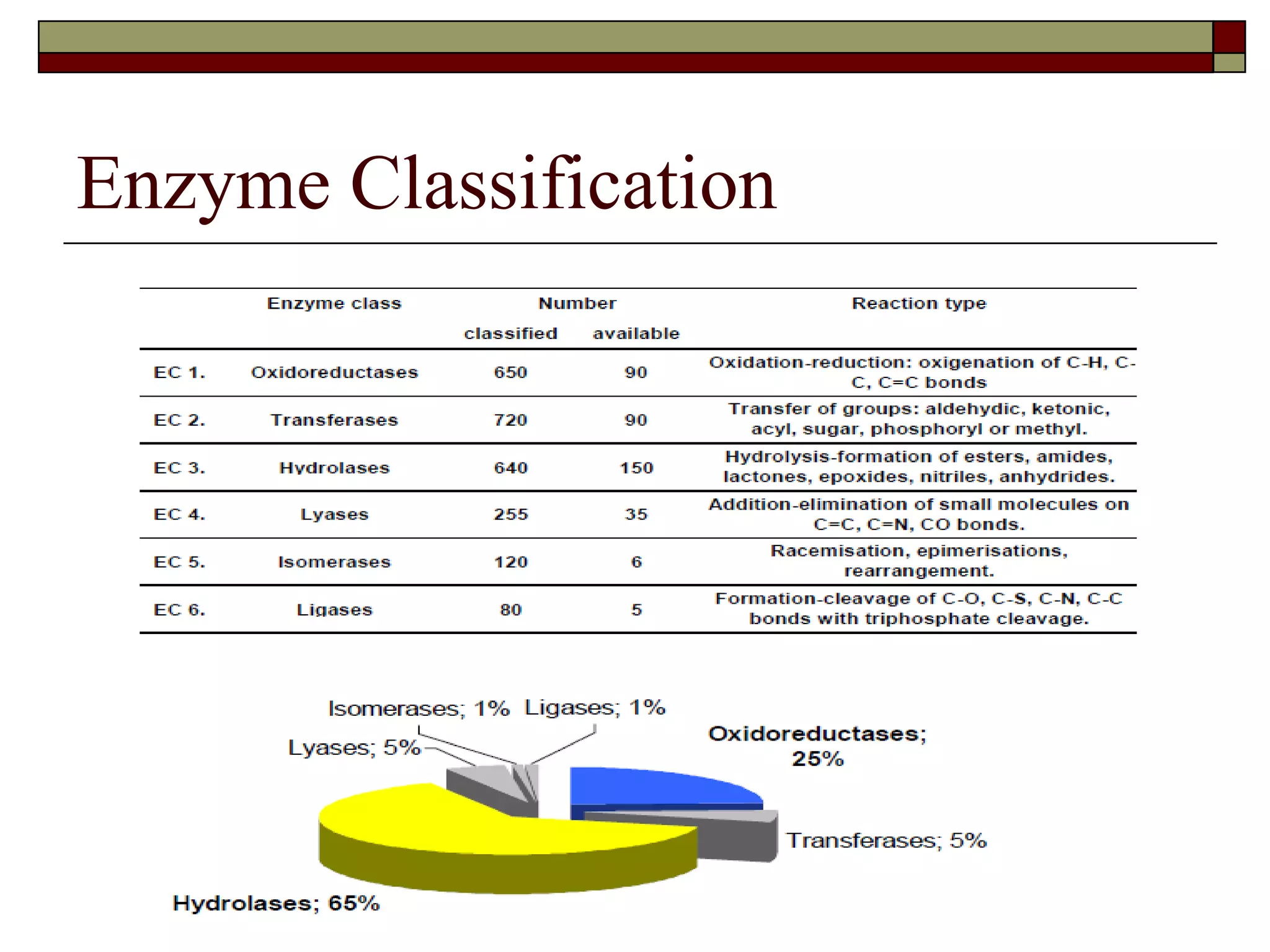
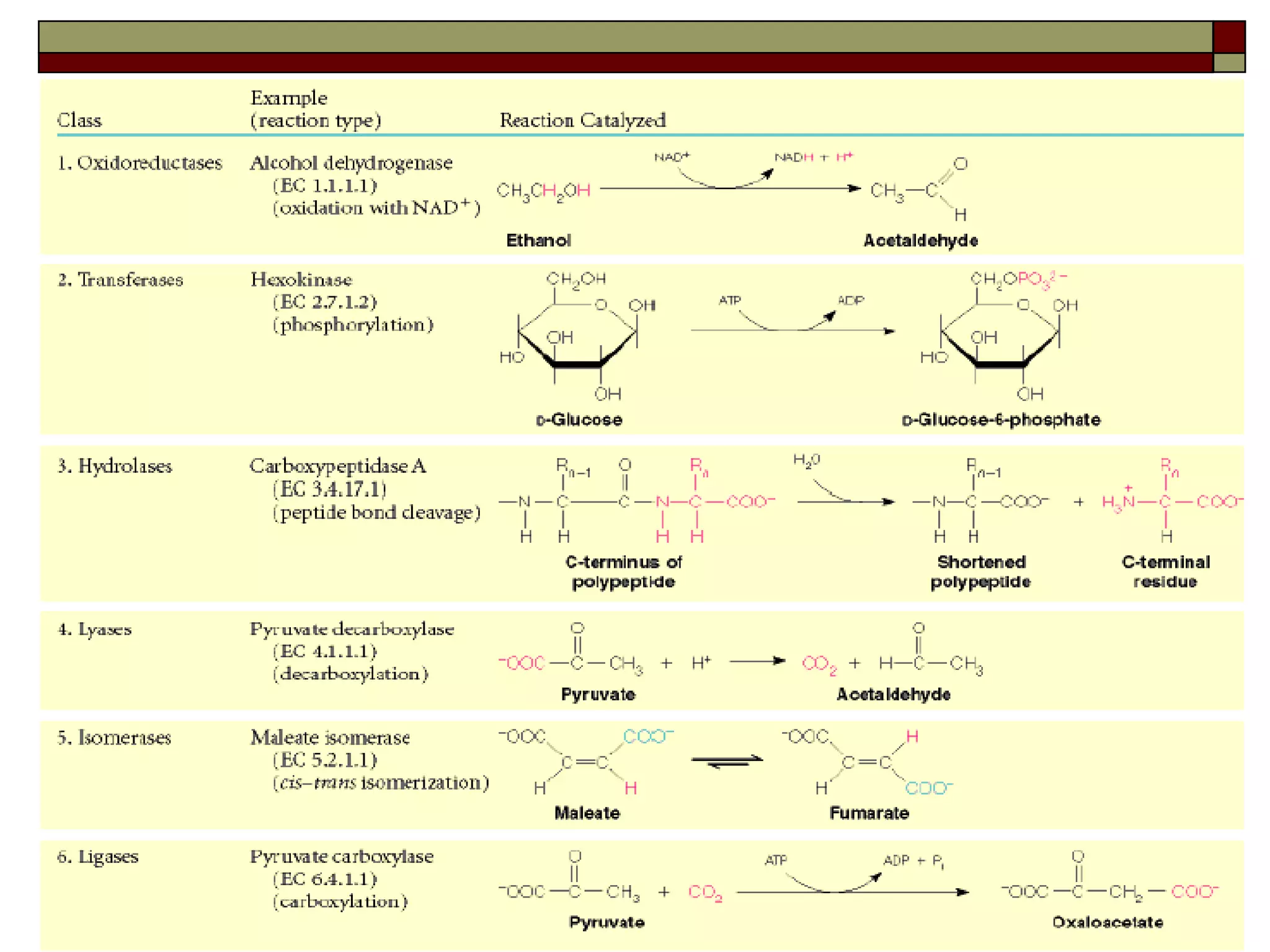
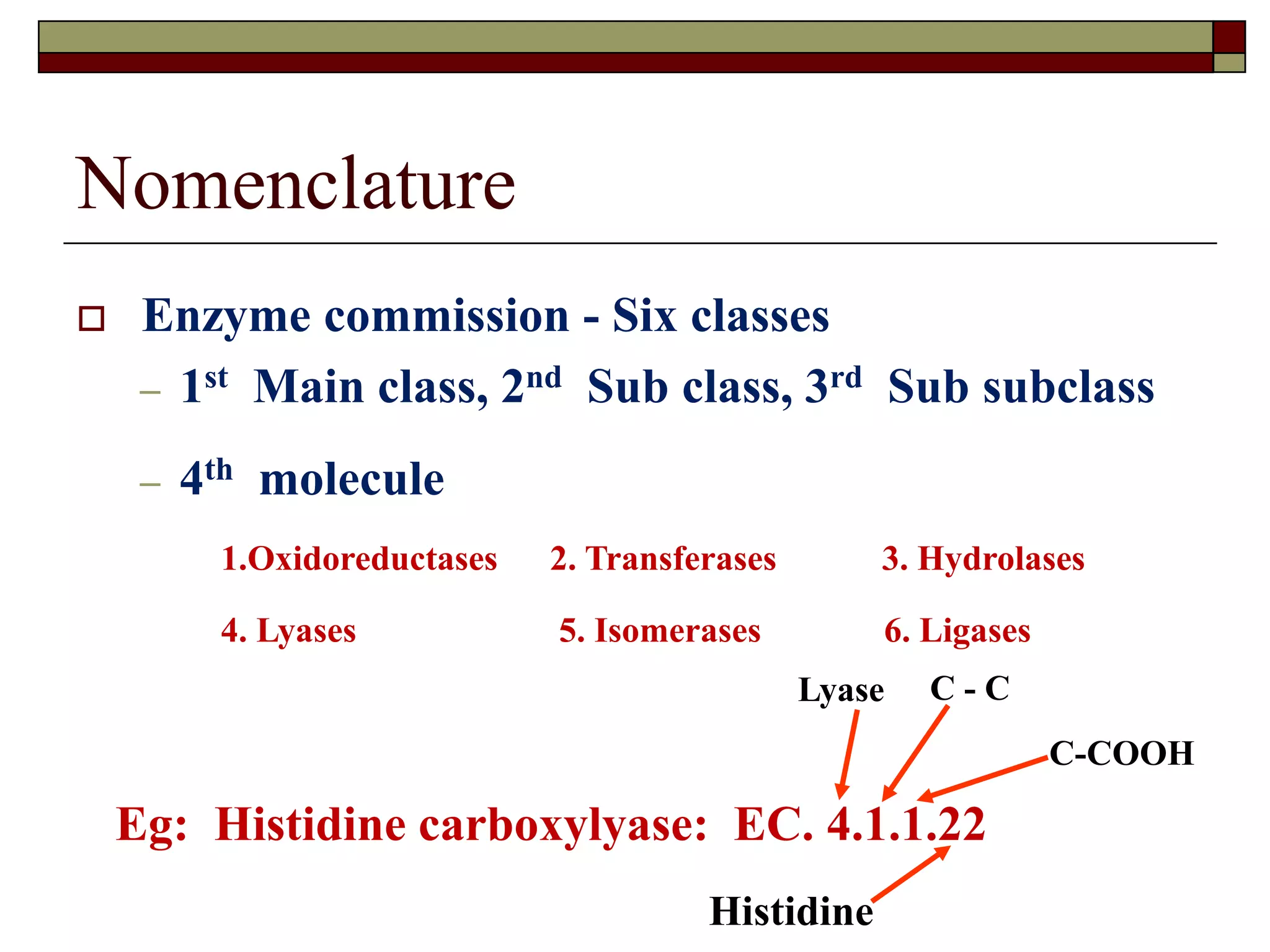
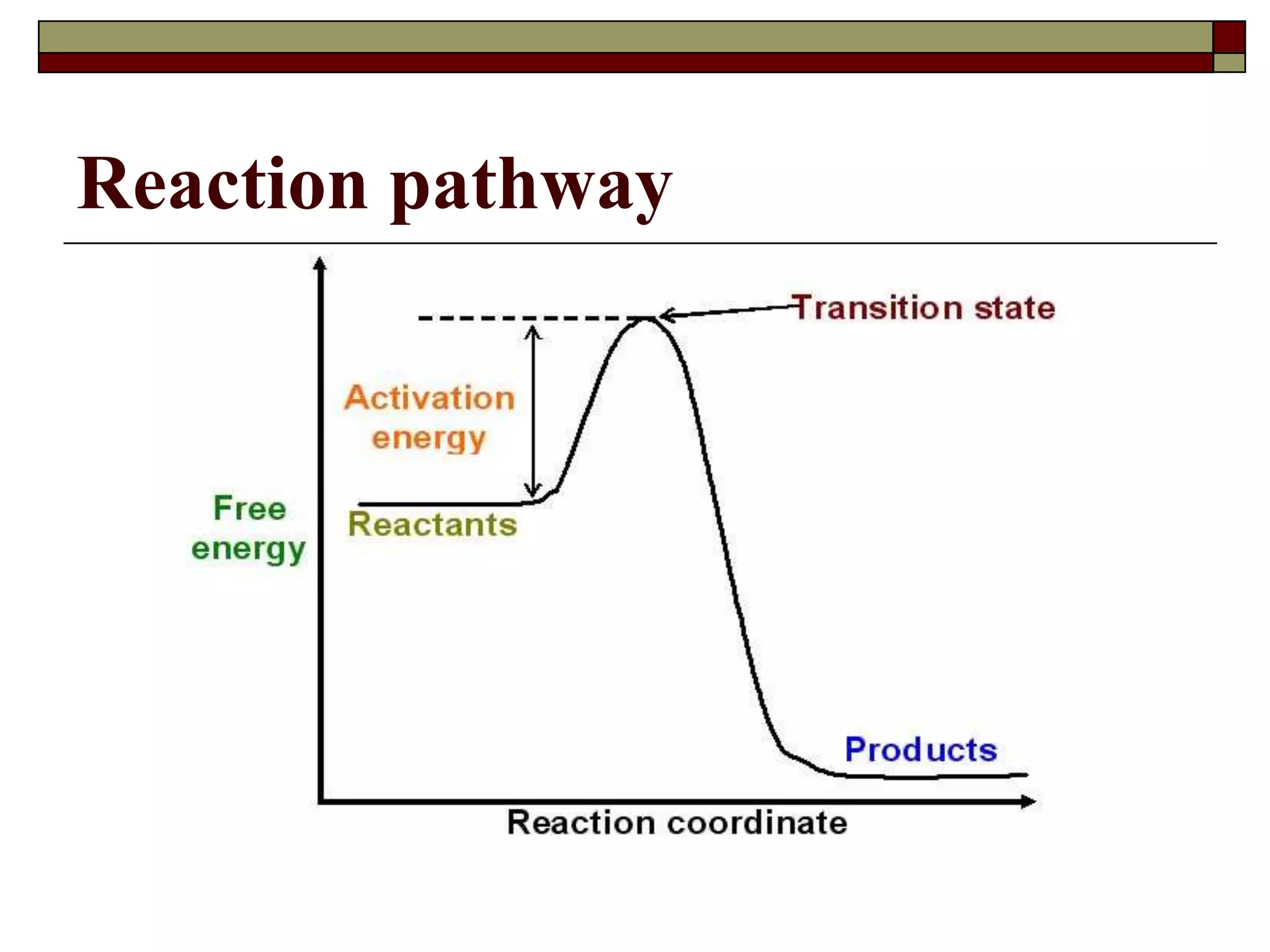
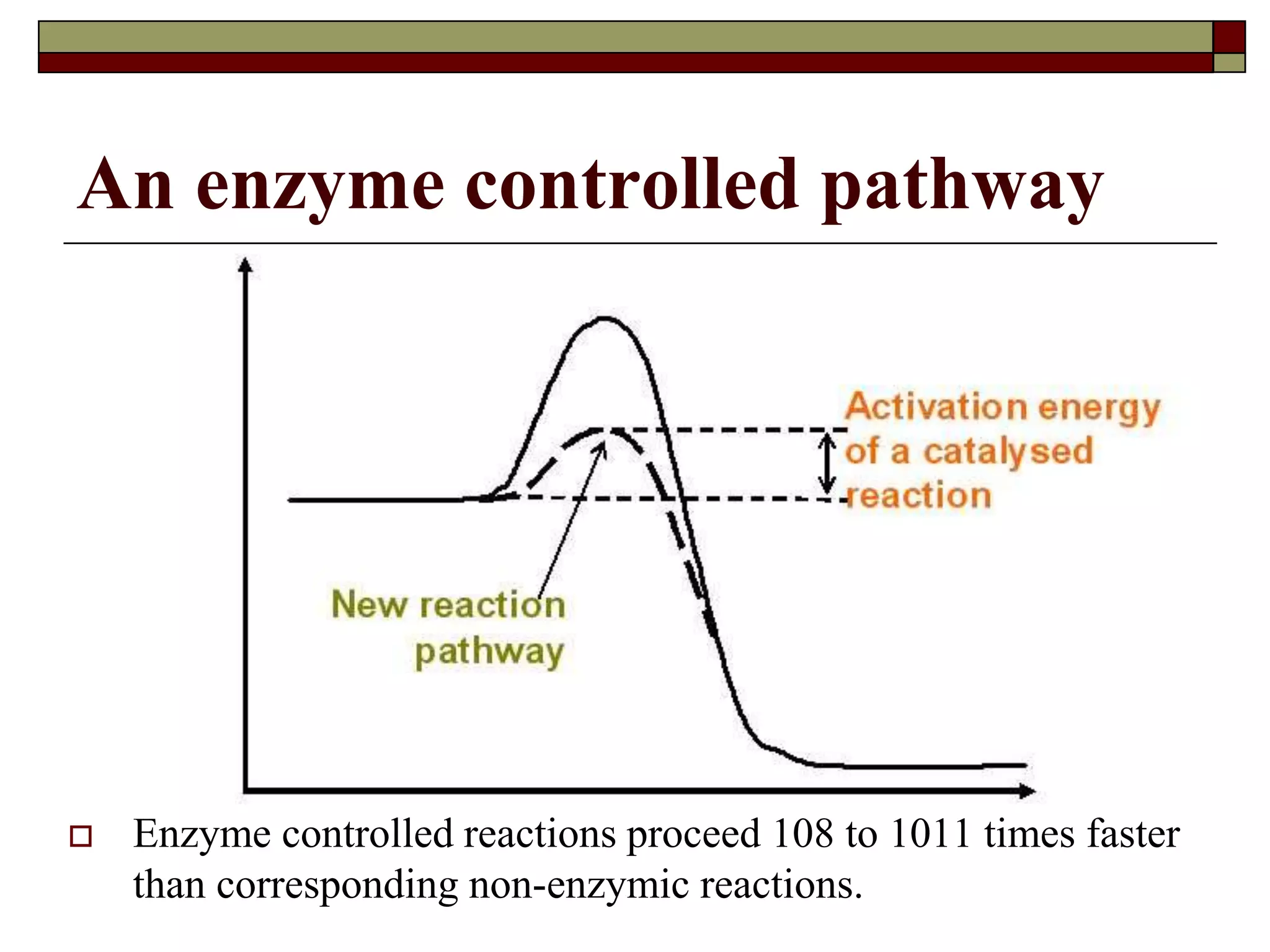
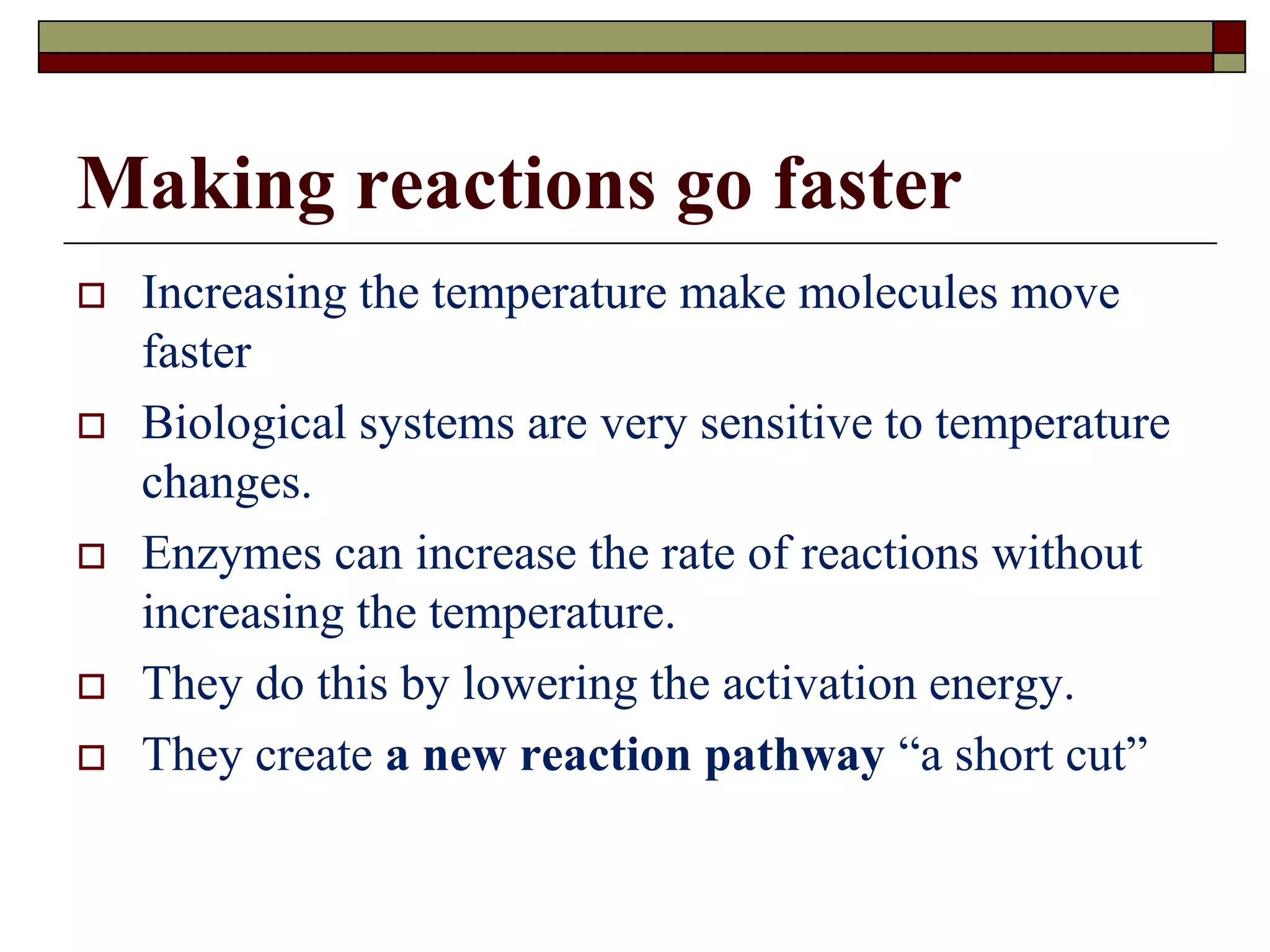
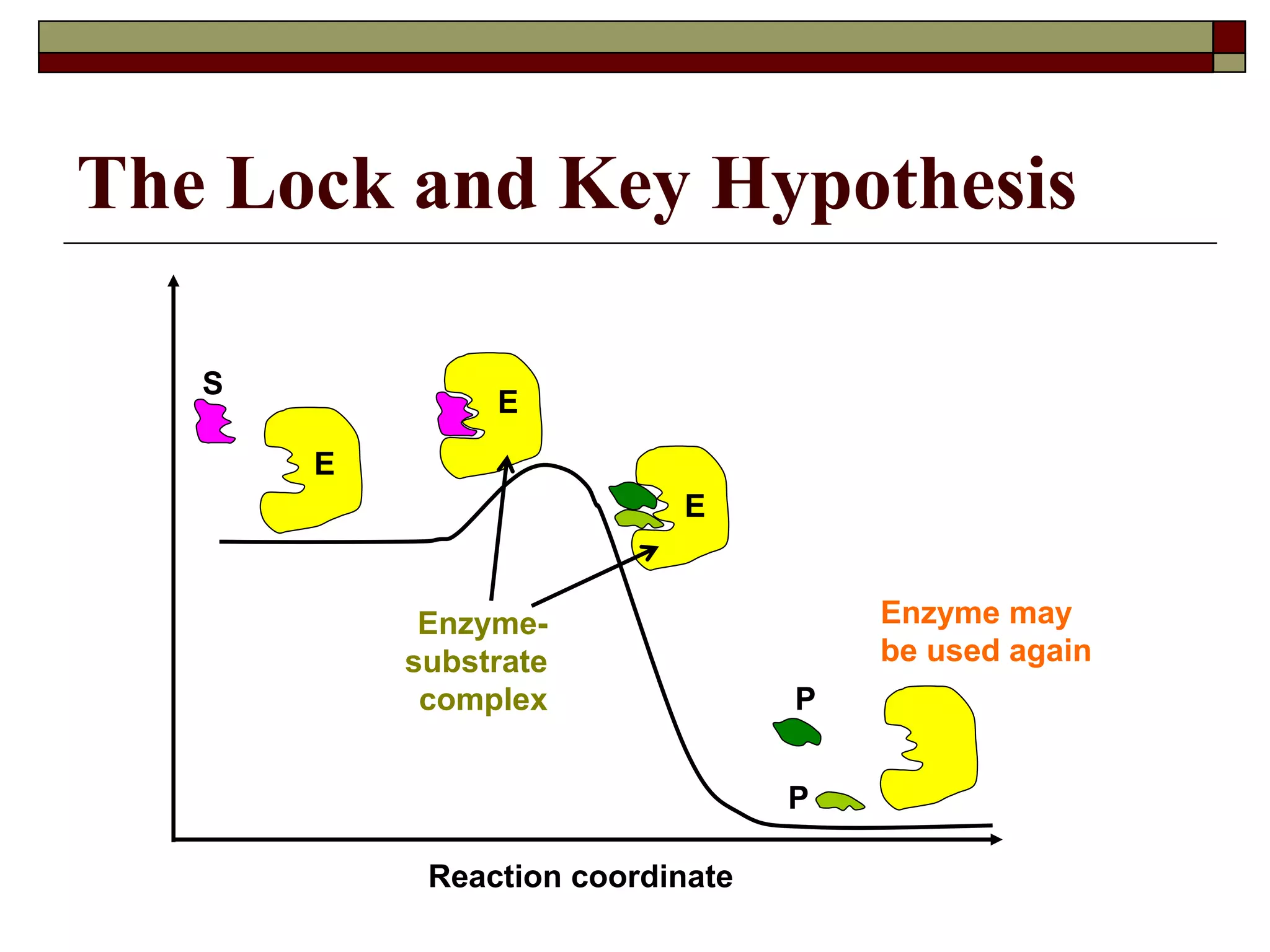
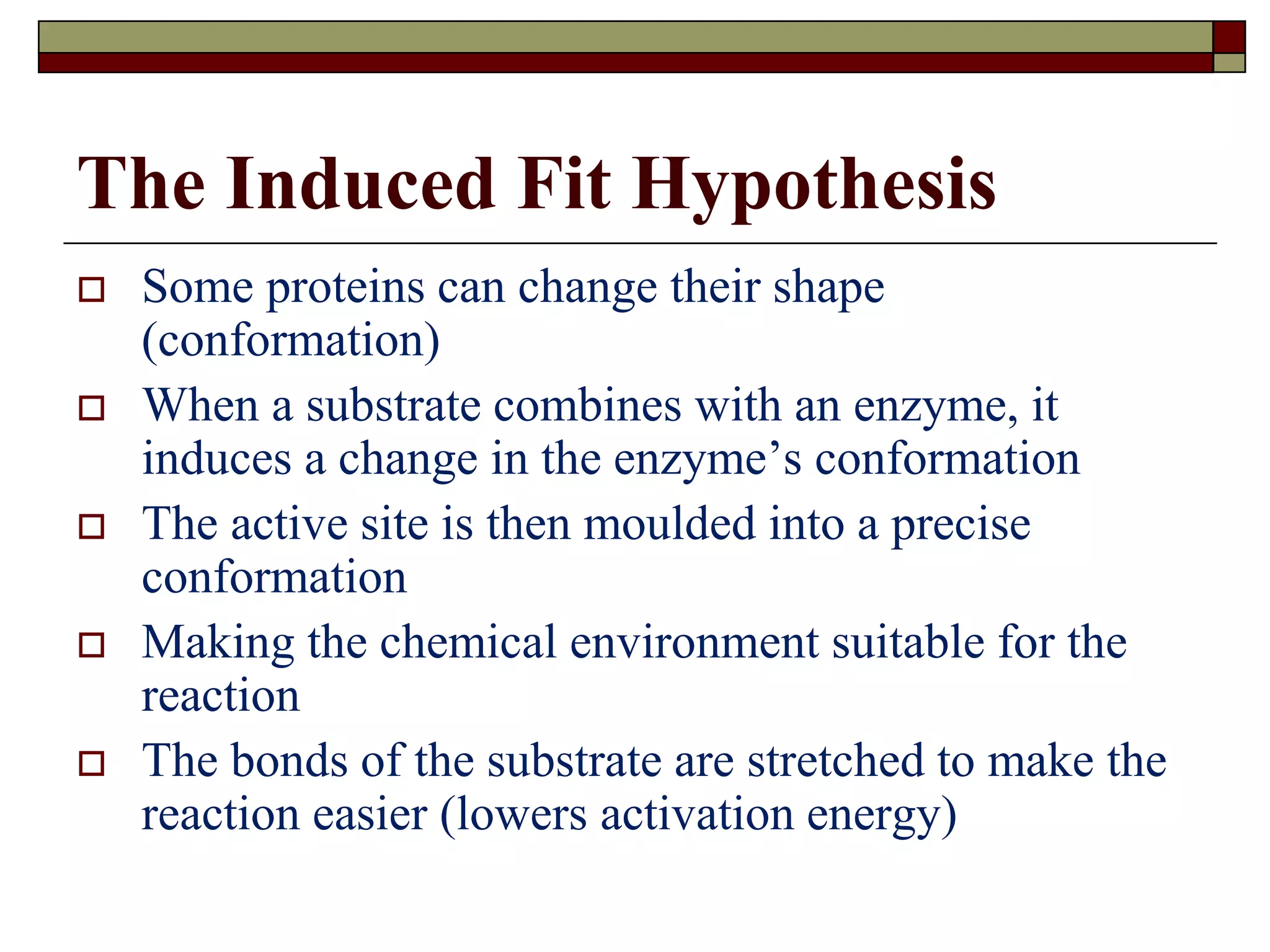
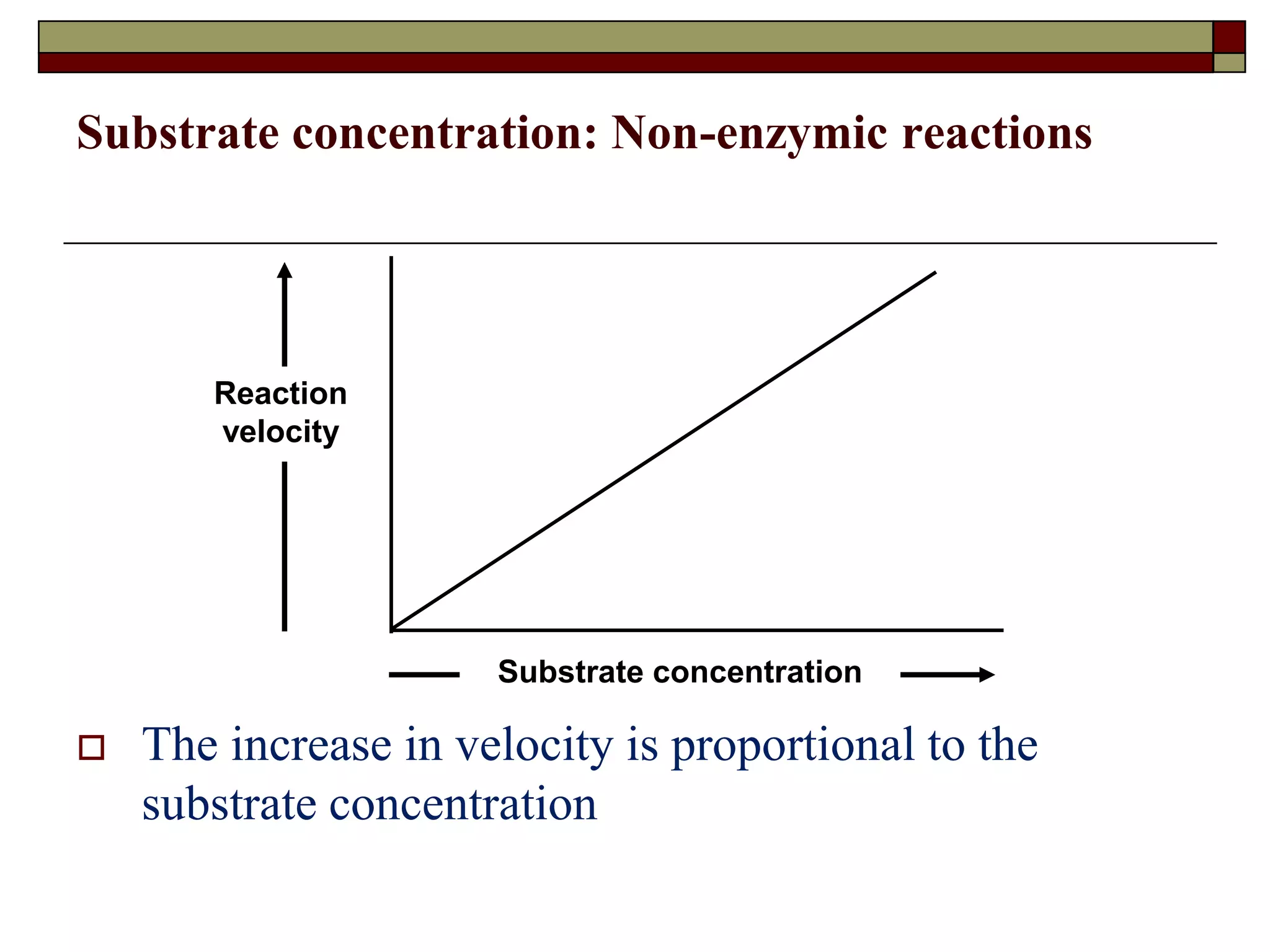
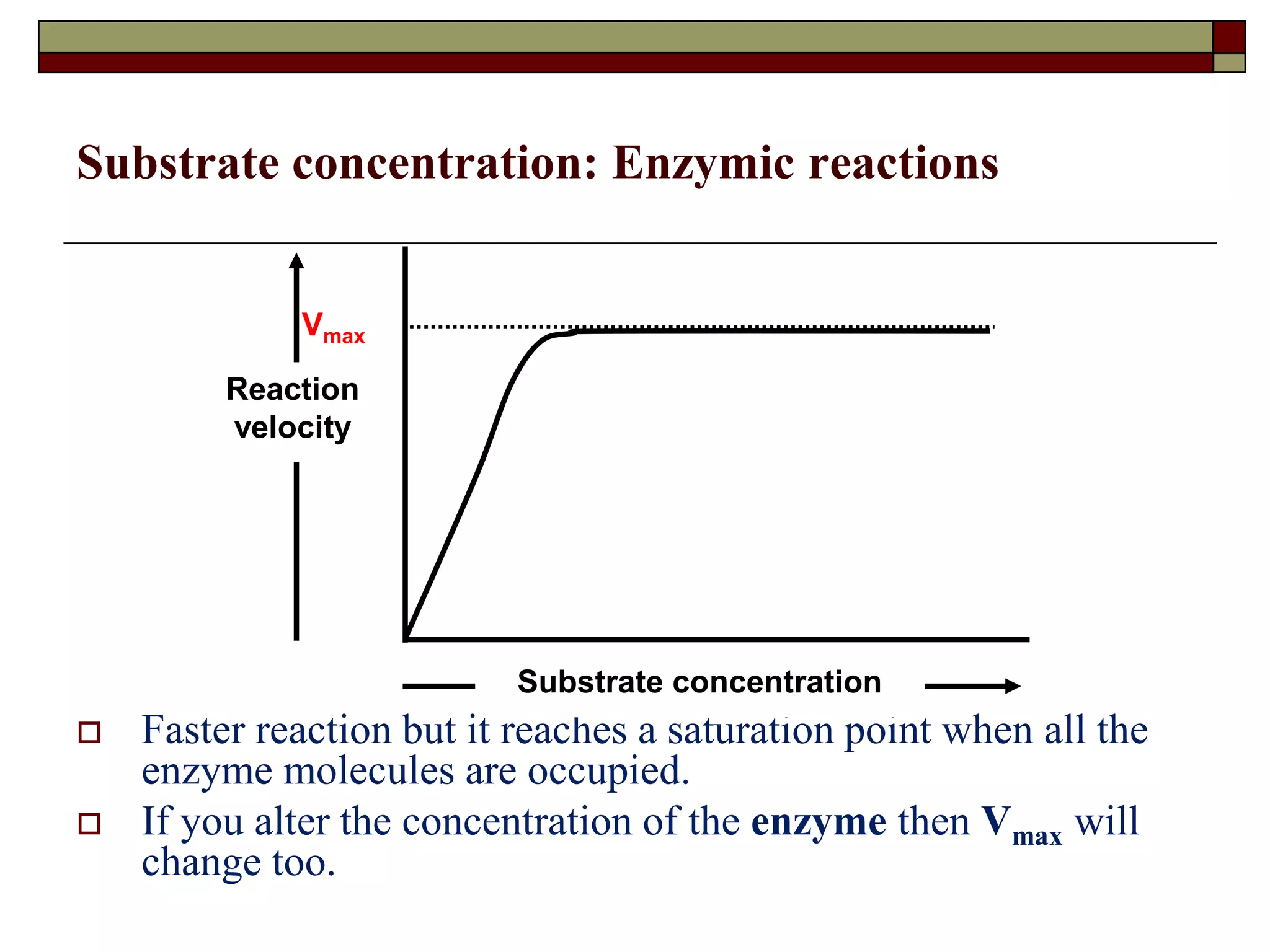
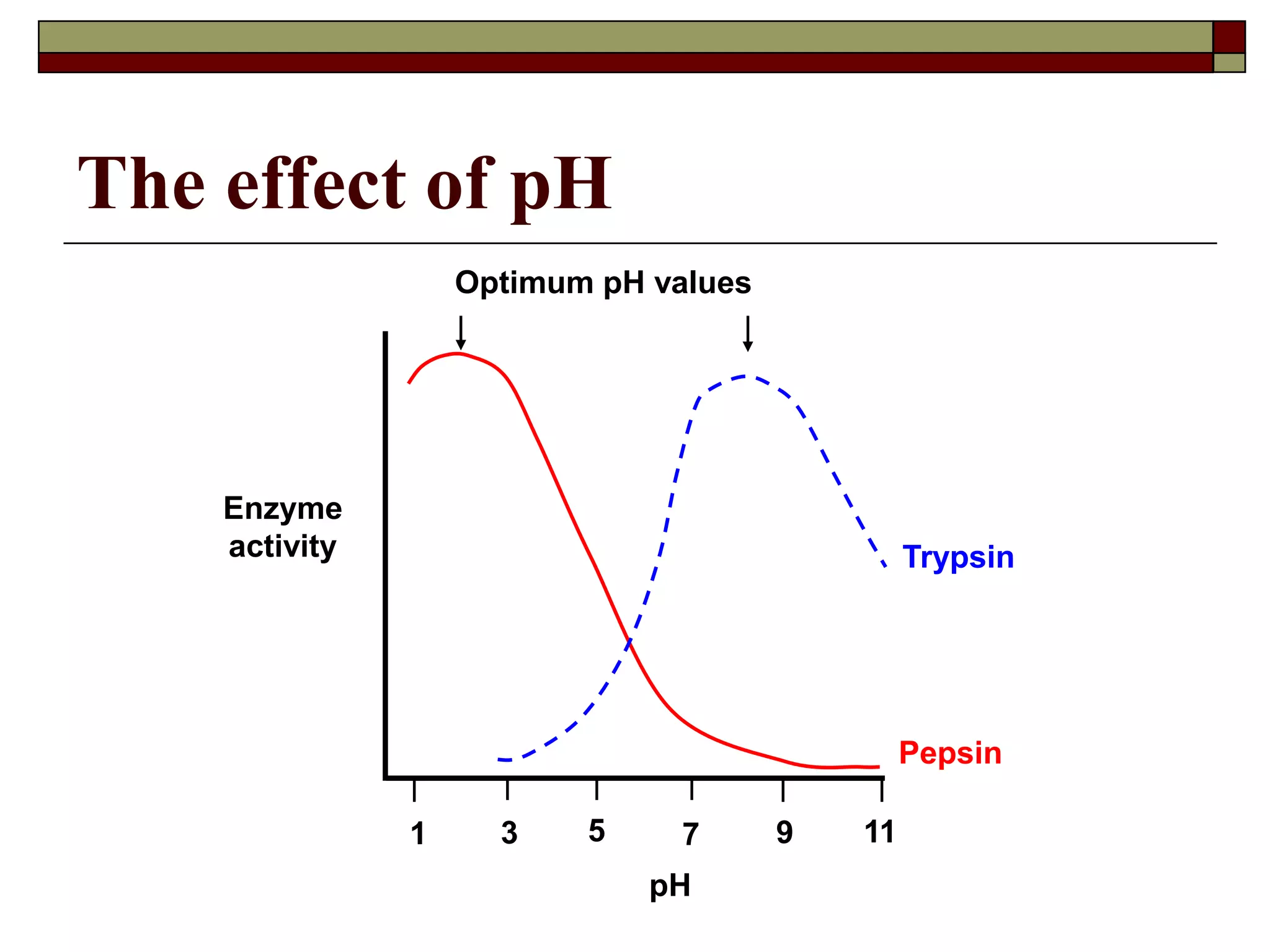
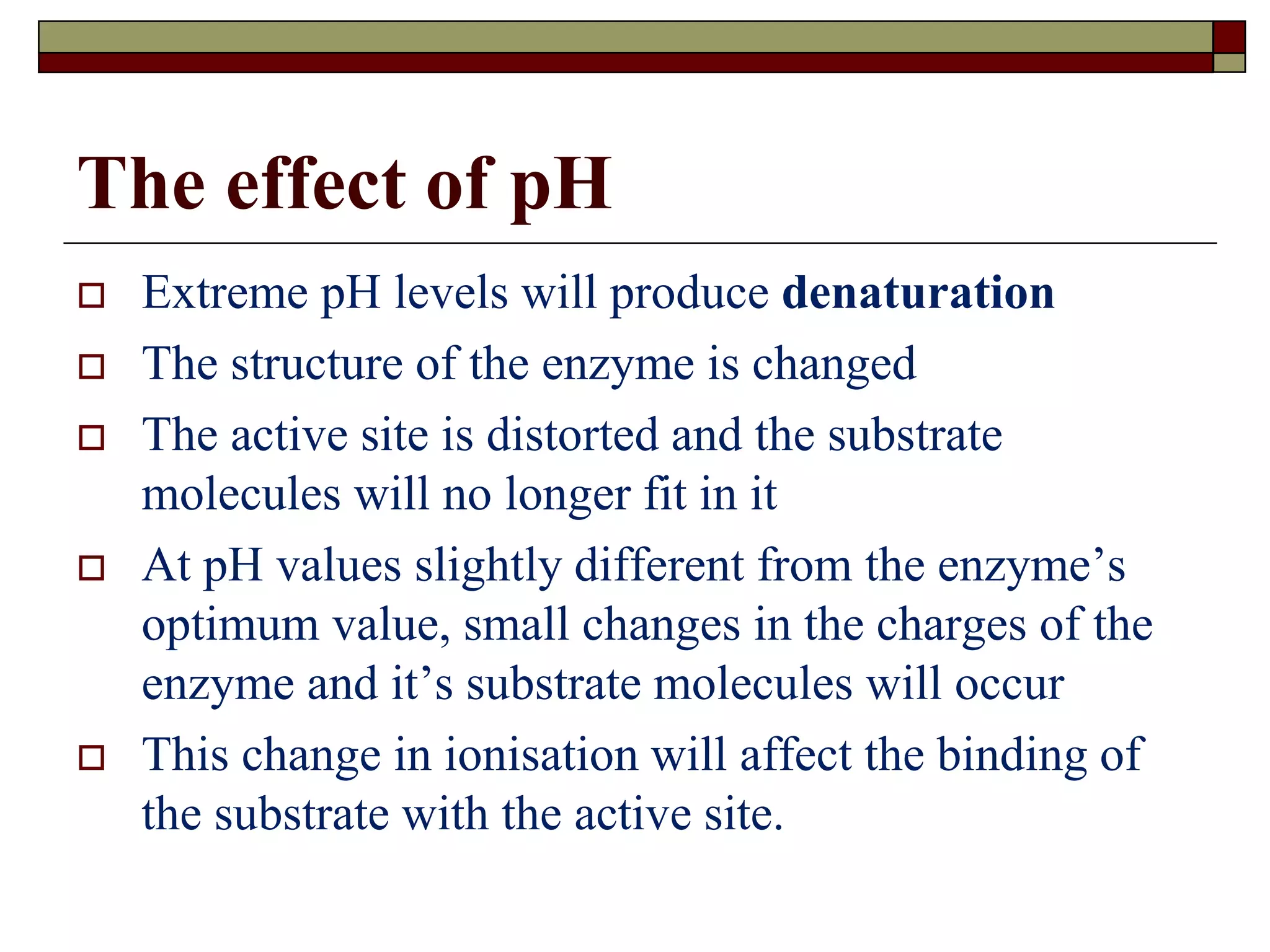
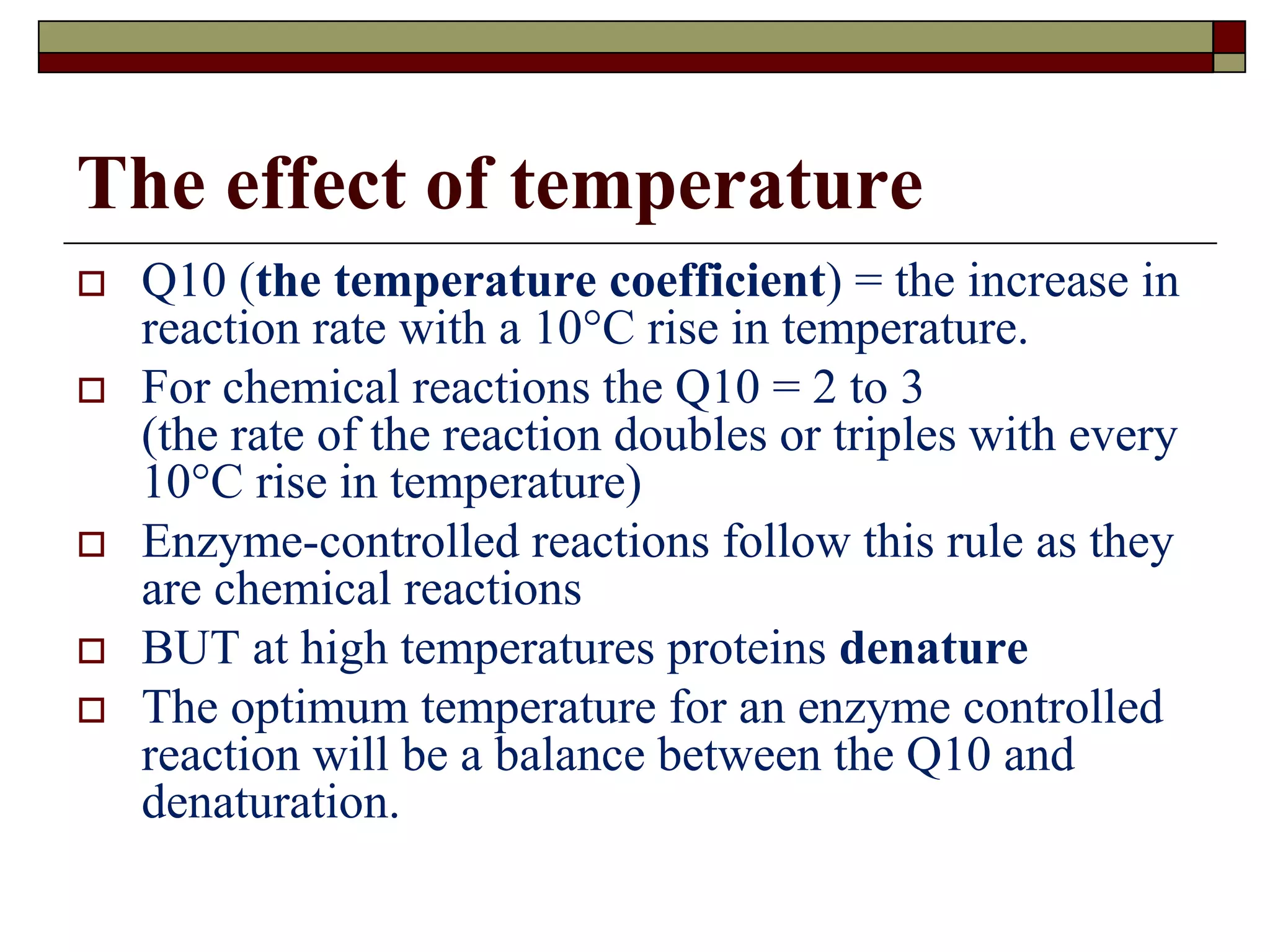
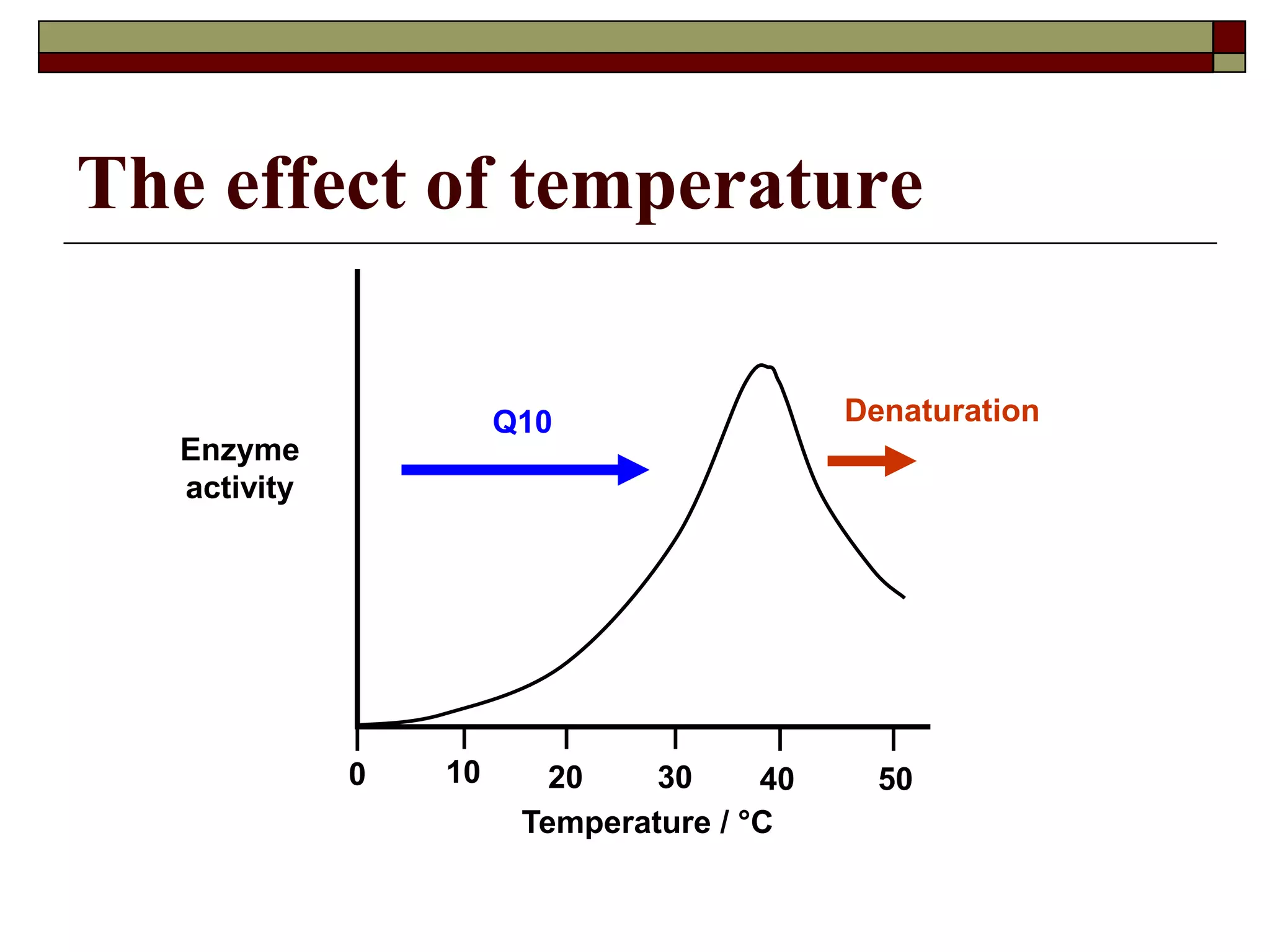
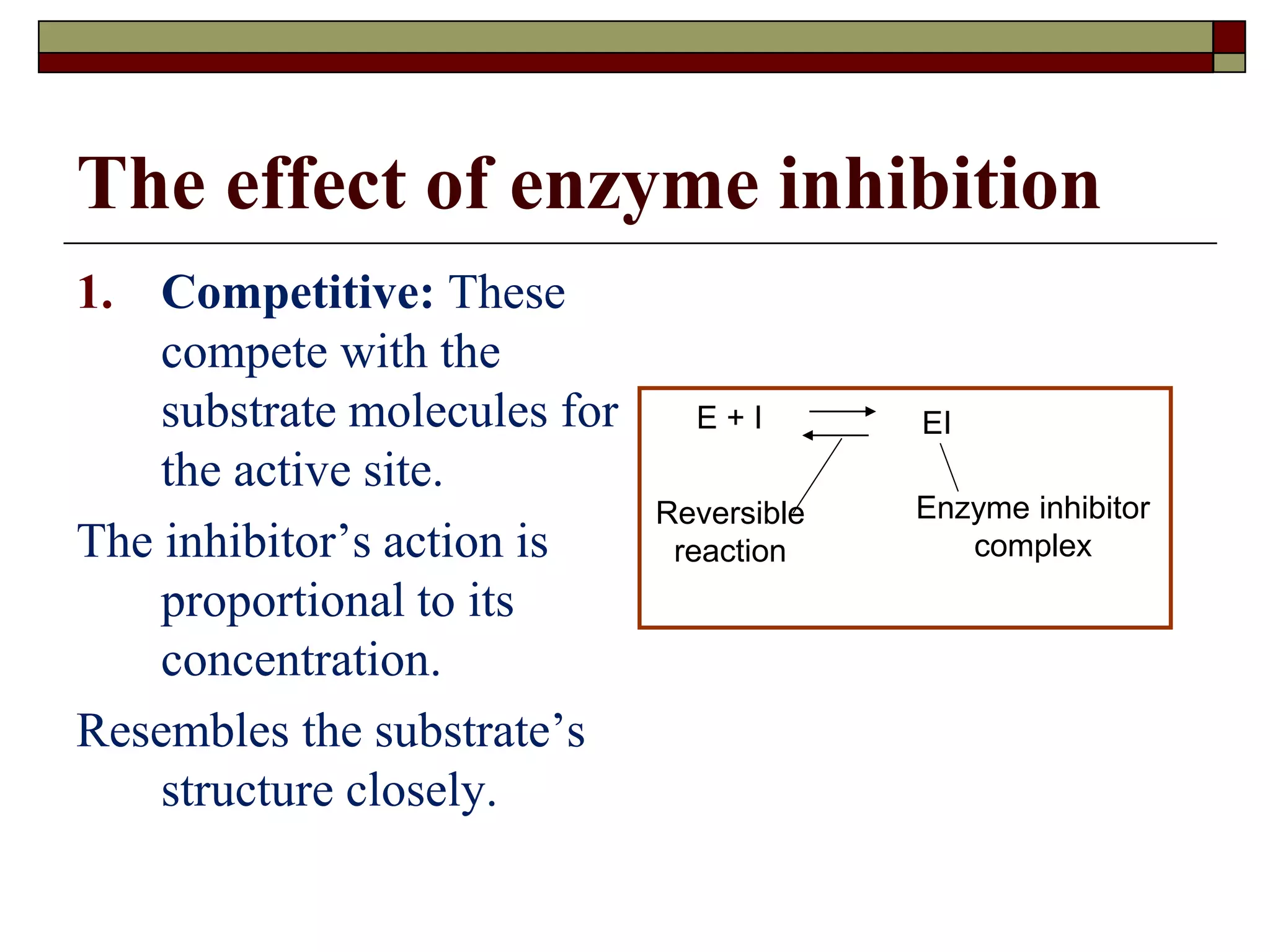
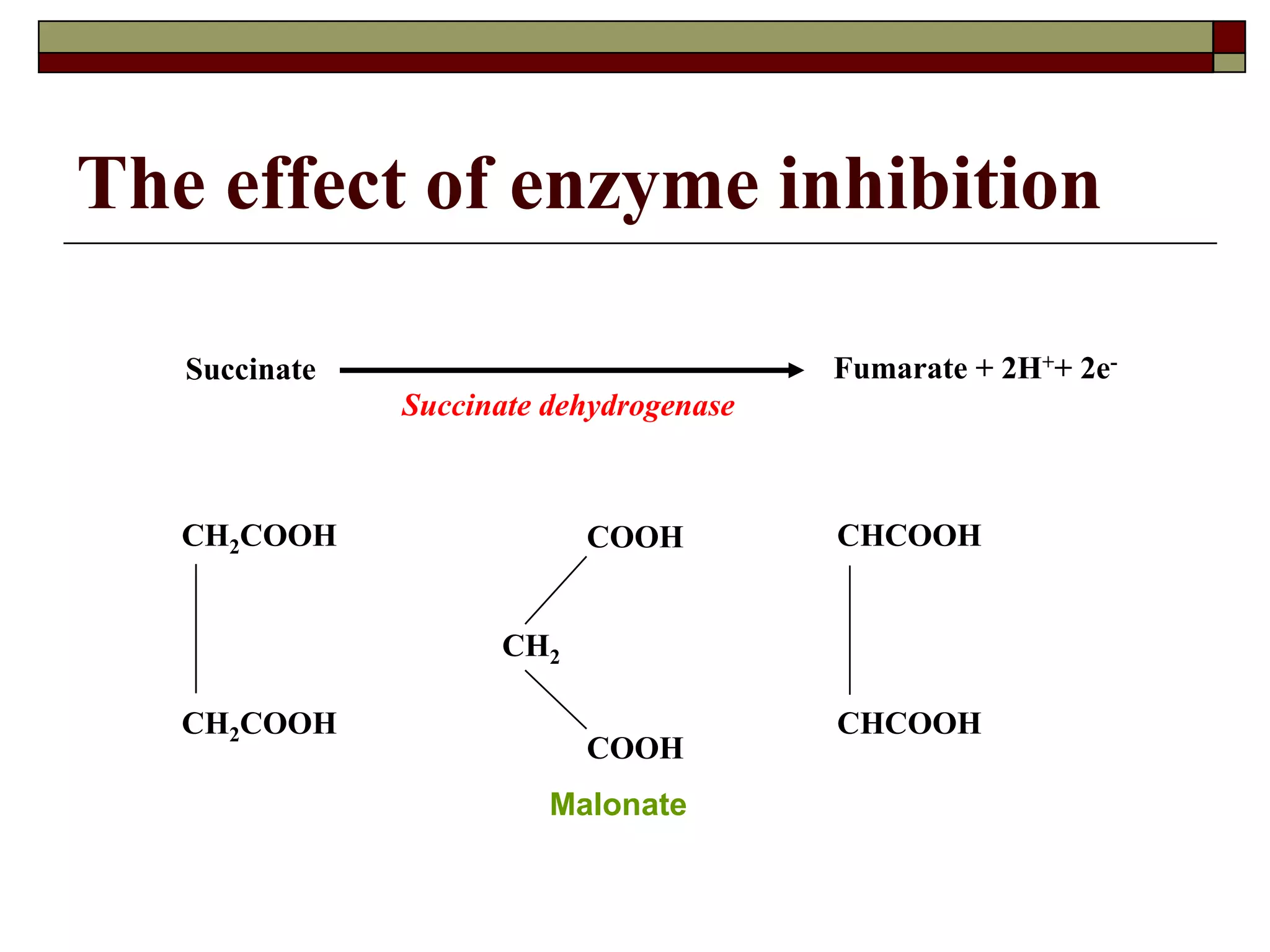
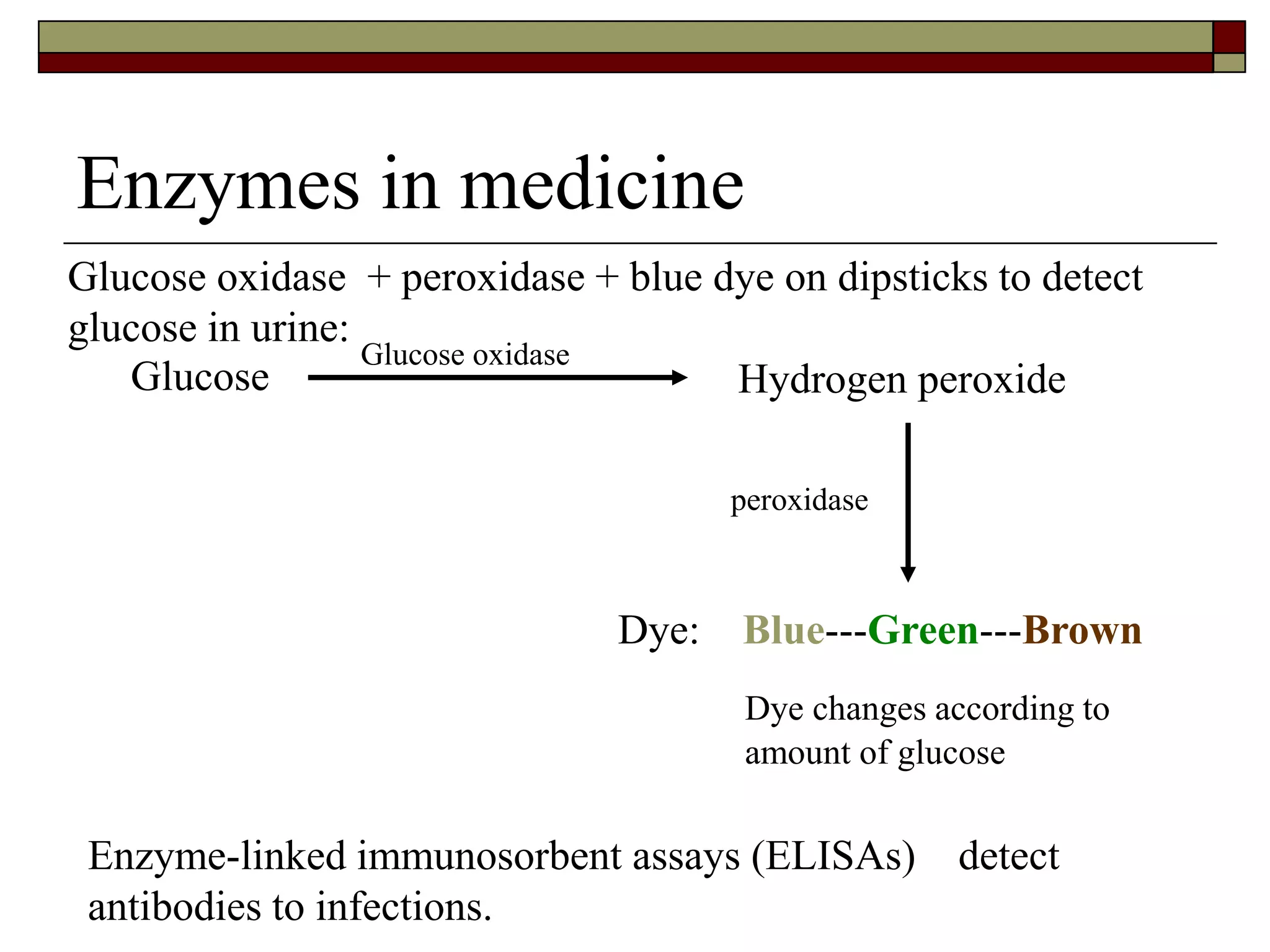
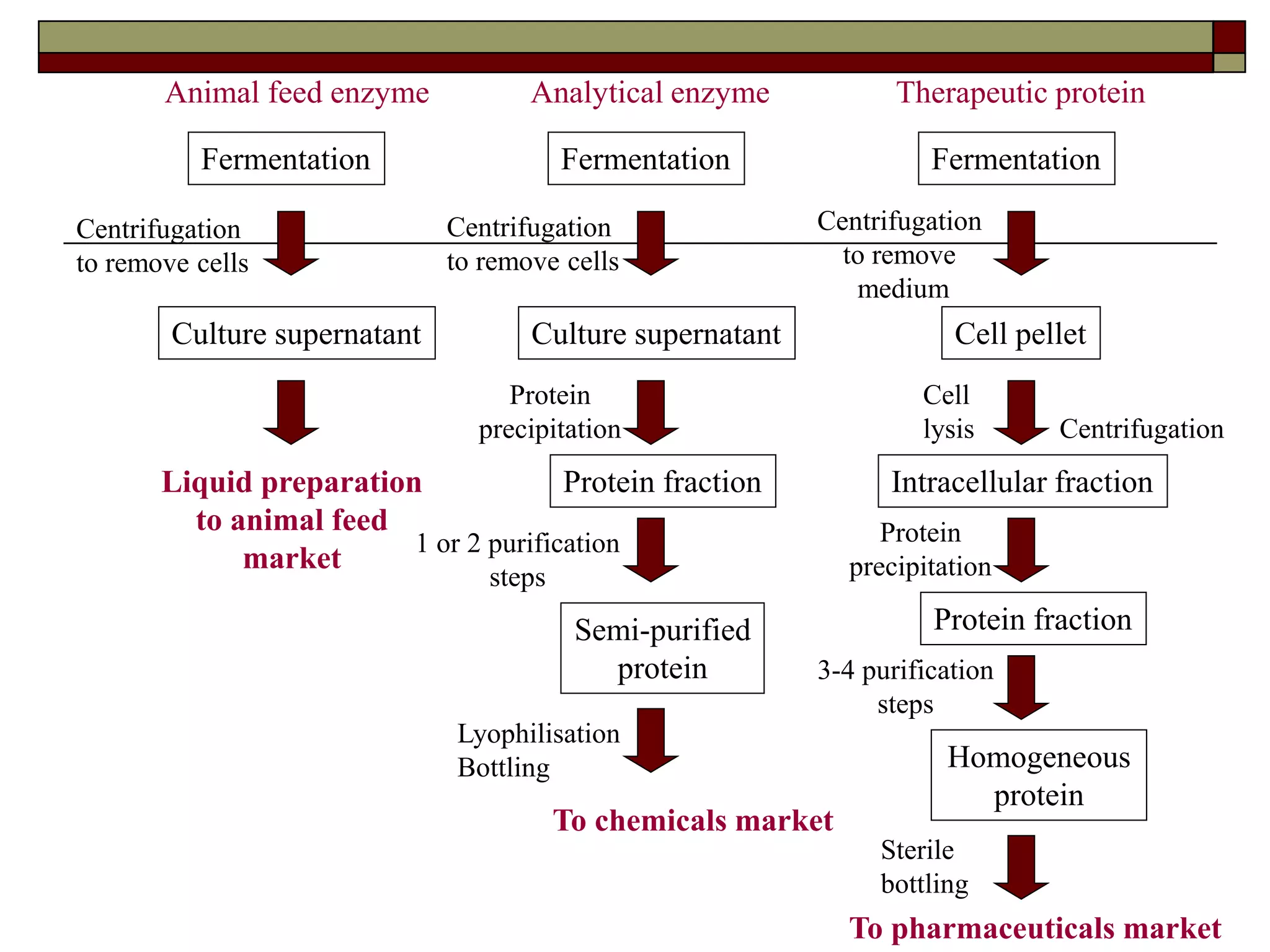
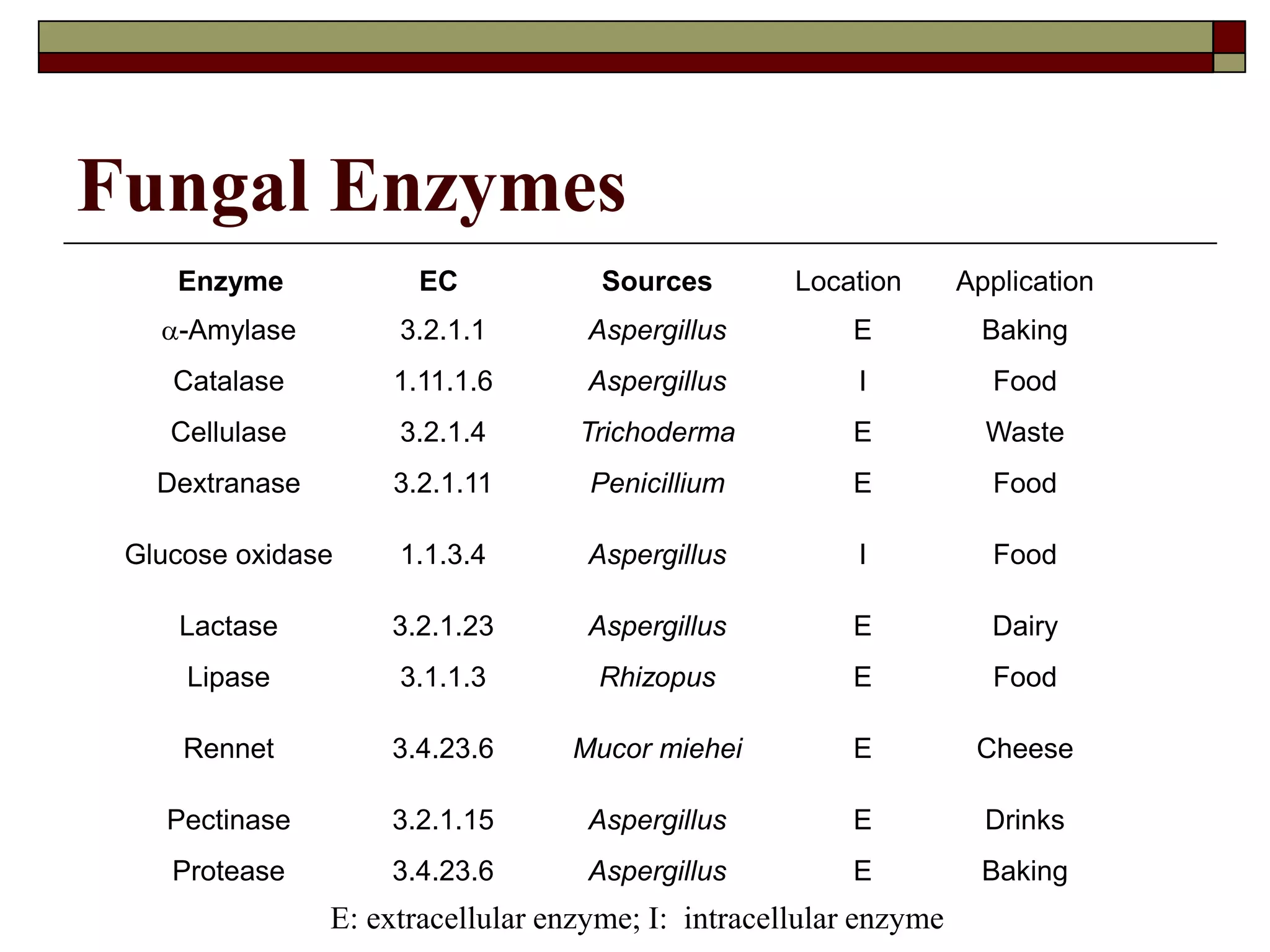
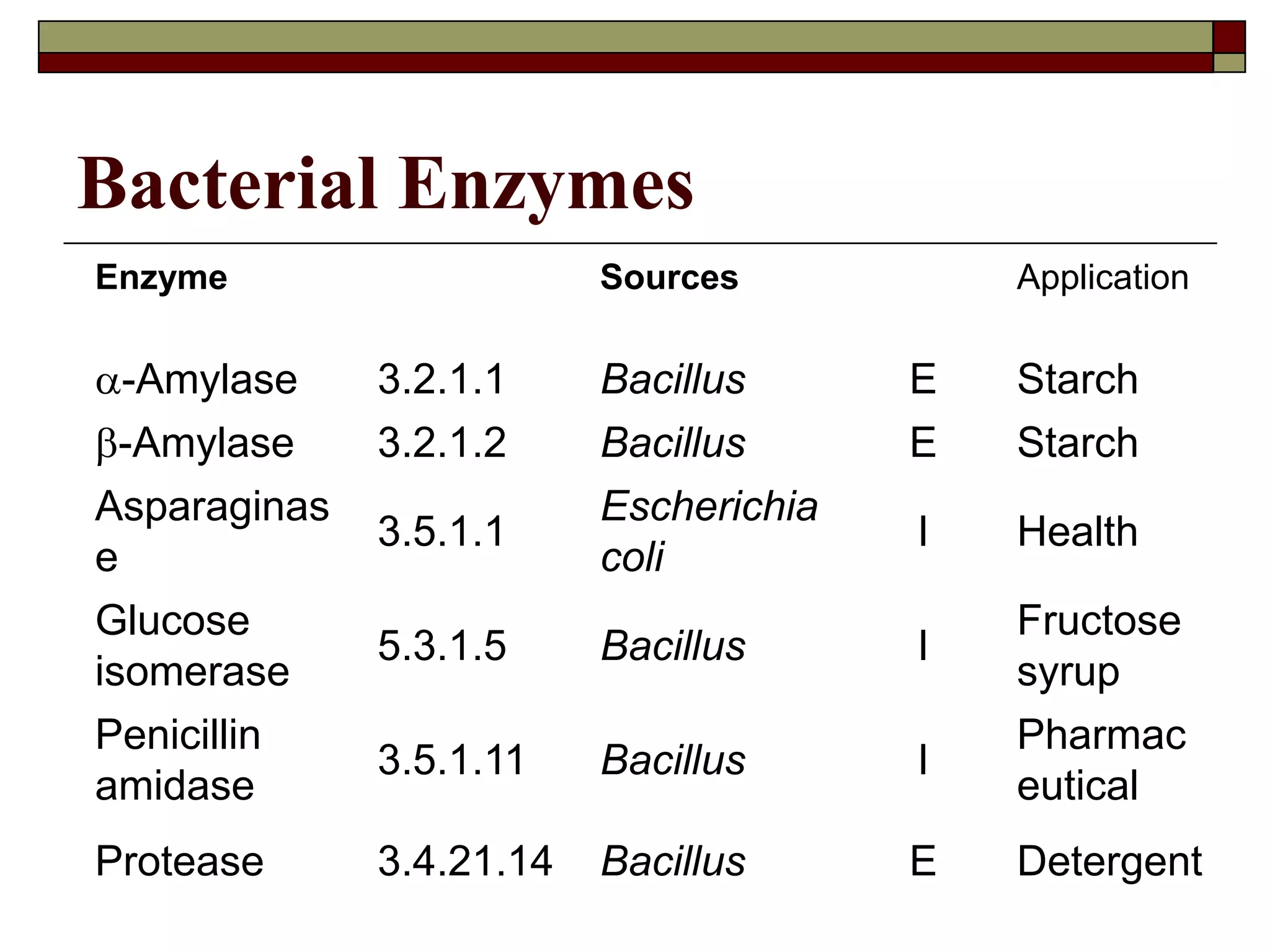
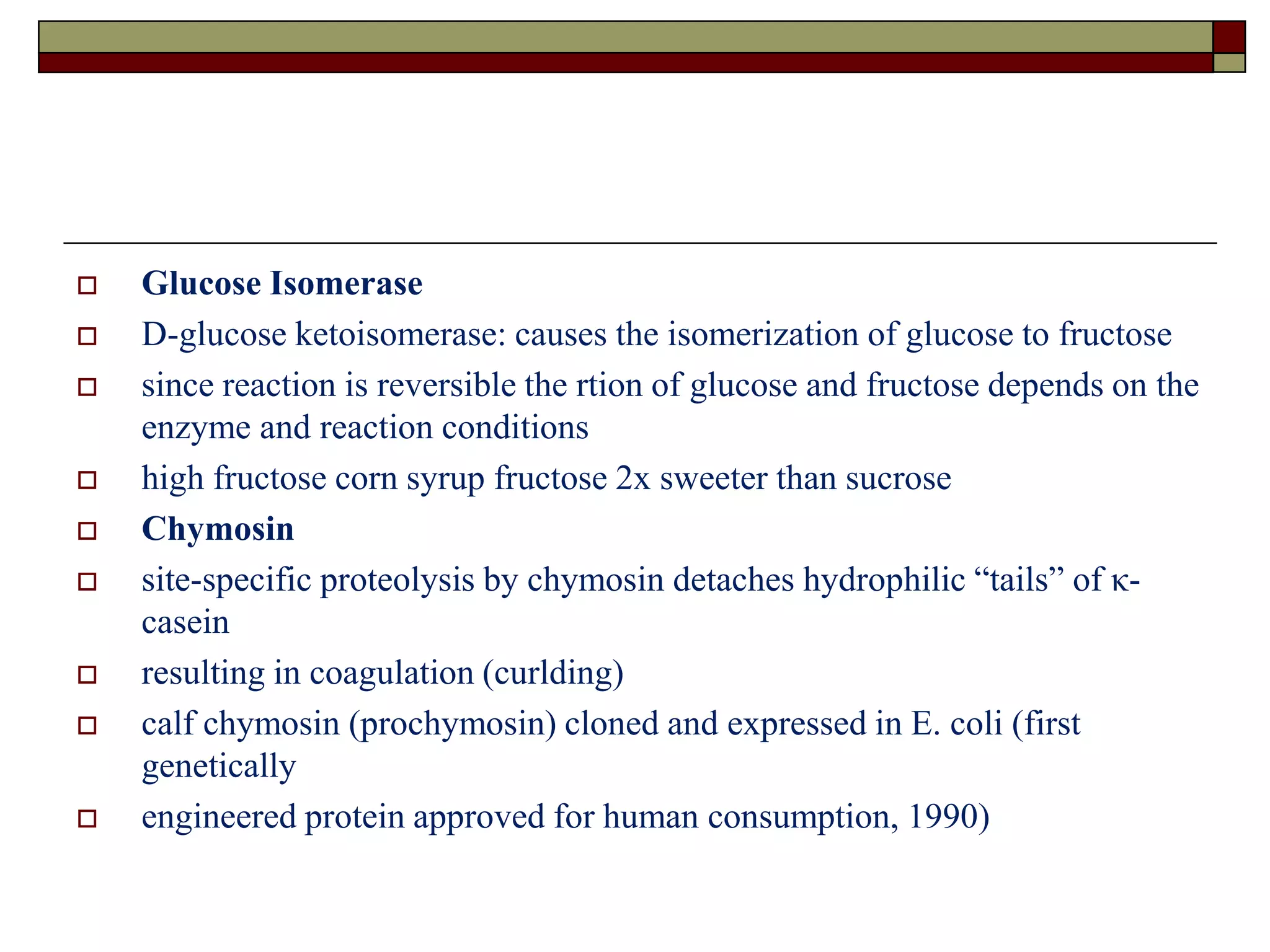
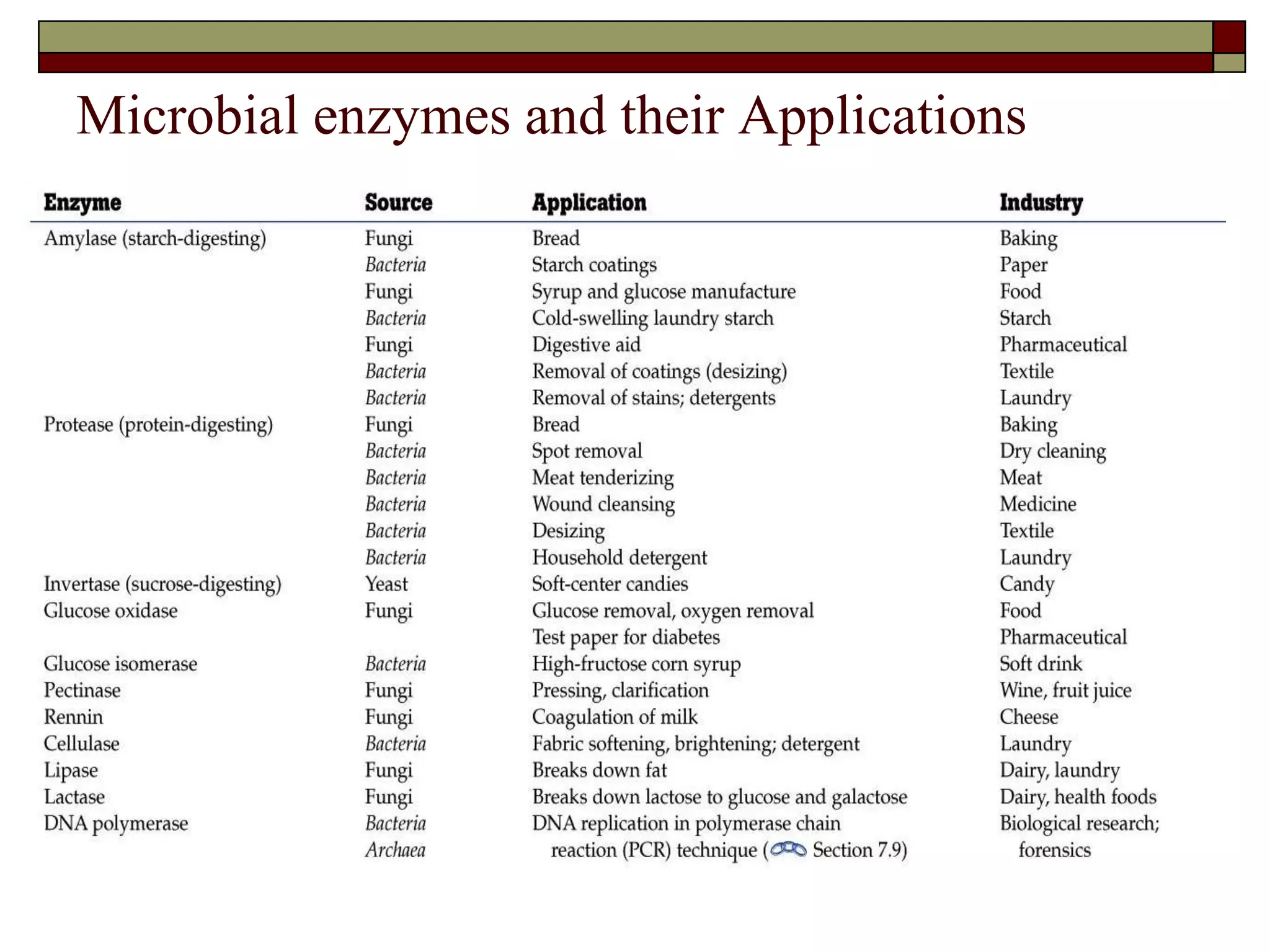
This document defines key terms and concepts related to enzymes. It begins by explaining that enzymes are protein complexes that lower the activation energy of biochemical reactions, accelerating their rate. It then provides definitions for important enzyme terminology, such as substrate, active site, cofactor, and zymogen. The document discusses several models that describe how enzymes catalyze reactions, including the lock-and-key and induced fit hypotheses. It also explains how factors like substrate concentration, pH, temperature, and inhibitors can influence enzyme activity. The key roles of enzymes in biological systems and various applications are also briefly outlined.