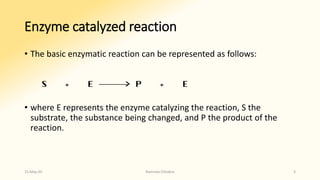
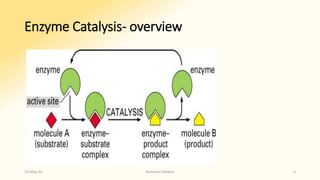
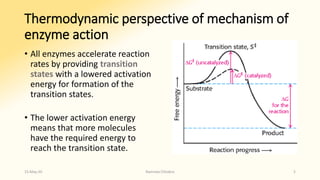
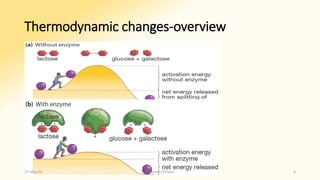
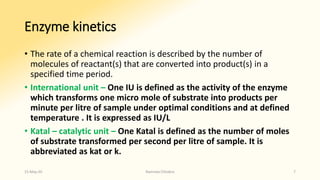
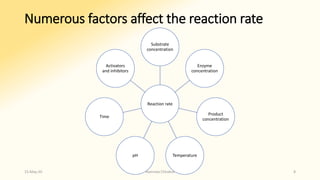
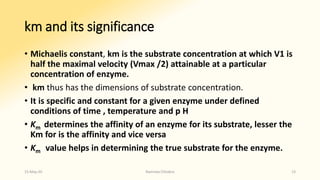
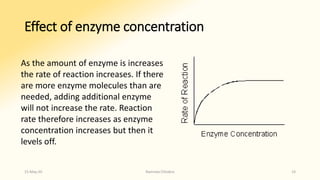
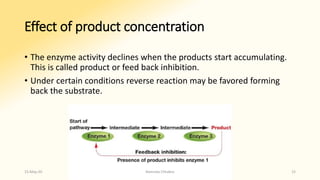
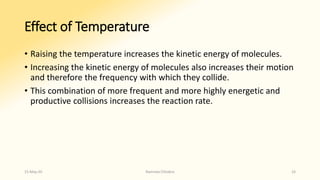
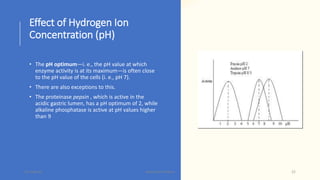
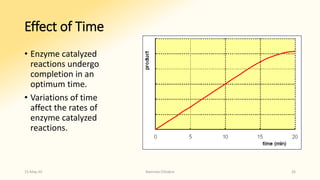
The document discusses factors that influence enzyme activity, notably substrate concentration, temperature, pH, and the presence of activators and inhibitors. It highlights concepts such as Michaelis constant (Km) and reaction kinetics, including first and zero order reactions. Additionally, it covers how enzyme concentrations and environmental conditions like temperature and pH optimize or inhibit enzymatic activity.



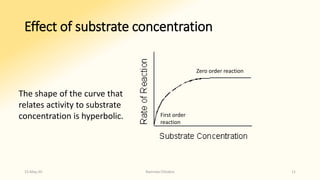
![• At points A or B, increasing
or decreasing [S] therefore
will increase or decrease the
number of ES complexes
with a corresponding change
in vi.
• The rate of reaction is
substrate dependent (First
order reaction).
• At point C essentially all the
enzyme is present as the ES
complex. Since no free
enzyme remains available for
forming ES, further increases
in [S] cannot increase the
rate of the reaction .
• Reaction rate therefore
becomes independent of
substrate concentration
(Zero order reaction).
15-May-20 Namrata Chhabra 12](https://image.slidesharecdn.com/factorsaffectingenzymeactivity-200515001130/85/Factors-affecting-enzyme-activity-12-320.jpg)


![Michaelis-Menten Kinetics
• The Michaelis-Menten equation is a quantitative description of the
relationship among the rate of an enzyme- catalyzed reaction [v1],
the concentration of substrate [S] and two constants, Vmax and km
(which are set by the particular equation).
The symbols used in the Michaelis-
Menten equation refer to the reaction
rate [v1], maximum reaction rate (V
max), substrate concentration [S] and
the Michaelis-Menten constant (km).
15-May-20 Namrata Chhabra 27](https://image.slidesharecdn.com/factorsaffectingenzymeactivity-200515001130/85/Factors-affecting-enzyme-activity-27-320.jpg)
![Michaelis-Menten Kinetics
• The dependence of initial reaction velocity on [S] and Km may be
illustrated by evaluating the Michaelis-Menten equation under three
conditions.
• (1) When [S] is much less than km, the term km + [S] is essentially
equal to km. Replacing Km + [S] with Km reduces equation to
15-May-20 Namrata Chhabra 28](https://image.slidesharecdn.com/factorsaffectingenzymeactivity-200515001130/85/Factors-affecting-enzyme-activity-28-320.jpg)
![When [S] is
much less
than km
• Since V max and km are both constants, their
ratio is a constant (k).
• In other words, when [S] is considerably below
km, V max is proportionate to k[S].
• The initial reaction velocity therefore is directly
proportionate to [S].
15-May-20 Namrata Chhabra 29](https://image.slidesharecdn.com/factorsaffectingenzymeactivity-200515001130/85/Factors-affecting-enzyme-activity-29-320.jpg)
![When [S] is much greater than km
• ,the term km + [S] is essentially equal to [S]. Replacing km + [S] with
[S] reduces equation to :
Thus, when [S] greatly exceeds km, the reaction velocity is
maximal (V max) and unaffected by further increases in
substrate concentration.
15-May-20 Namrata Chhabra 30](https://image.slidesharecdn.com/factorsaffectingenzymeactivity-200515001130/85/Factors-affecting-enzyme-activity-30-320.jpg)
![(3) When [S] = km
• Equation states that when [S] equals km, the initial velocity
is half-maximal.
• Equation also reveals that km can be determined
experimentally from—the substrate concentration at which
the initial velocity is half-maximal.
15-May-20 Namrata Chhabra 31](https://image.slidesharecdn.com/factorsaffectingenzymeactivity-200515001130/85/Factors-affecting-enzyme-activity-31-320.jpg)

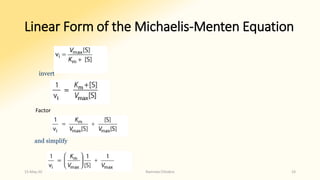
![Double reciprocal or Lineweaver-Burk plot
• Equation for a straight line, y = ax + b,
where y = 1/vi and x = 1/[S].
• A plot of 1/vi as y as a function of
1/[S] as x therefore gives a straight
line whose y intercept is 1/ V max and
whose slope is km / V max.
• Such a plot is called a double
reciprocal or Lineweaver-Burk plot
15-May-20 Namrata Chhabra 34](https://image.slidesharecdn.com/factorsaffectingenzymeactivity-200515001130/85/Factors-affecting-enzyme-activity-34-320.jpg)

