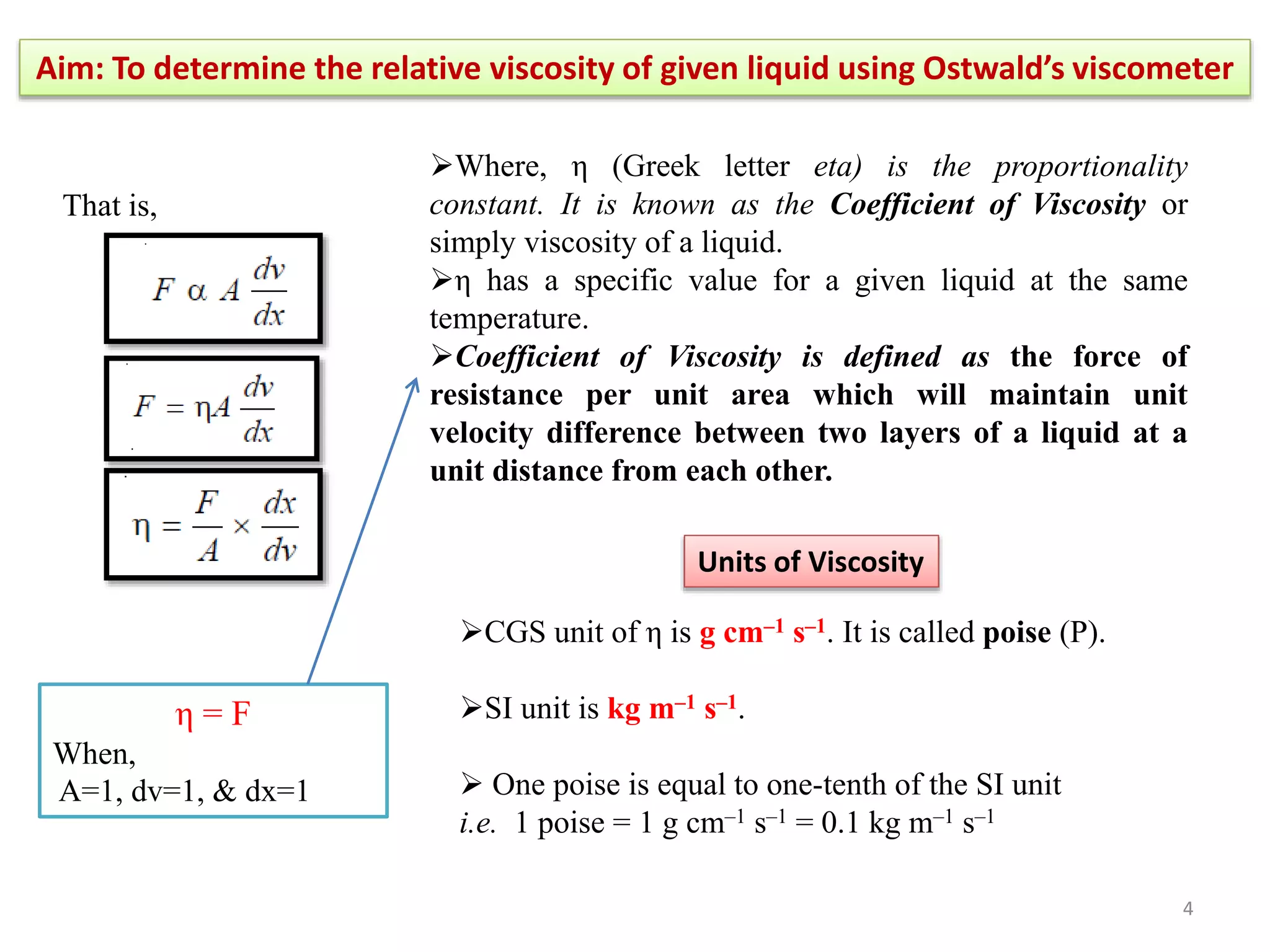
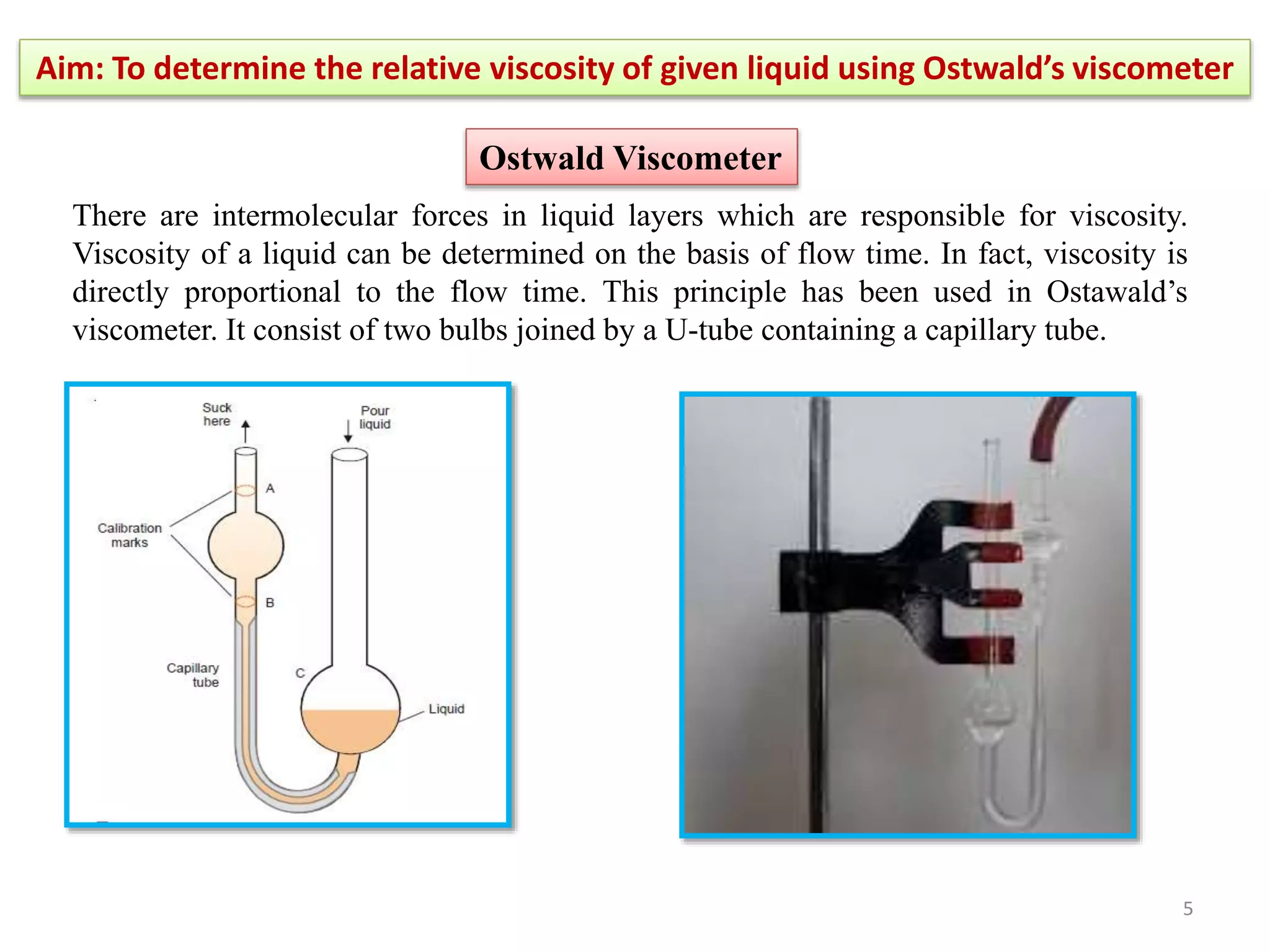
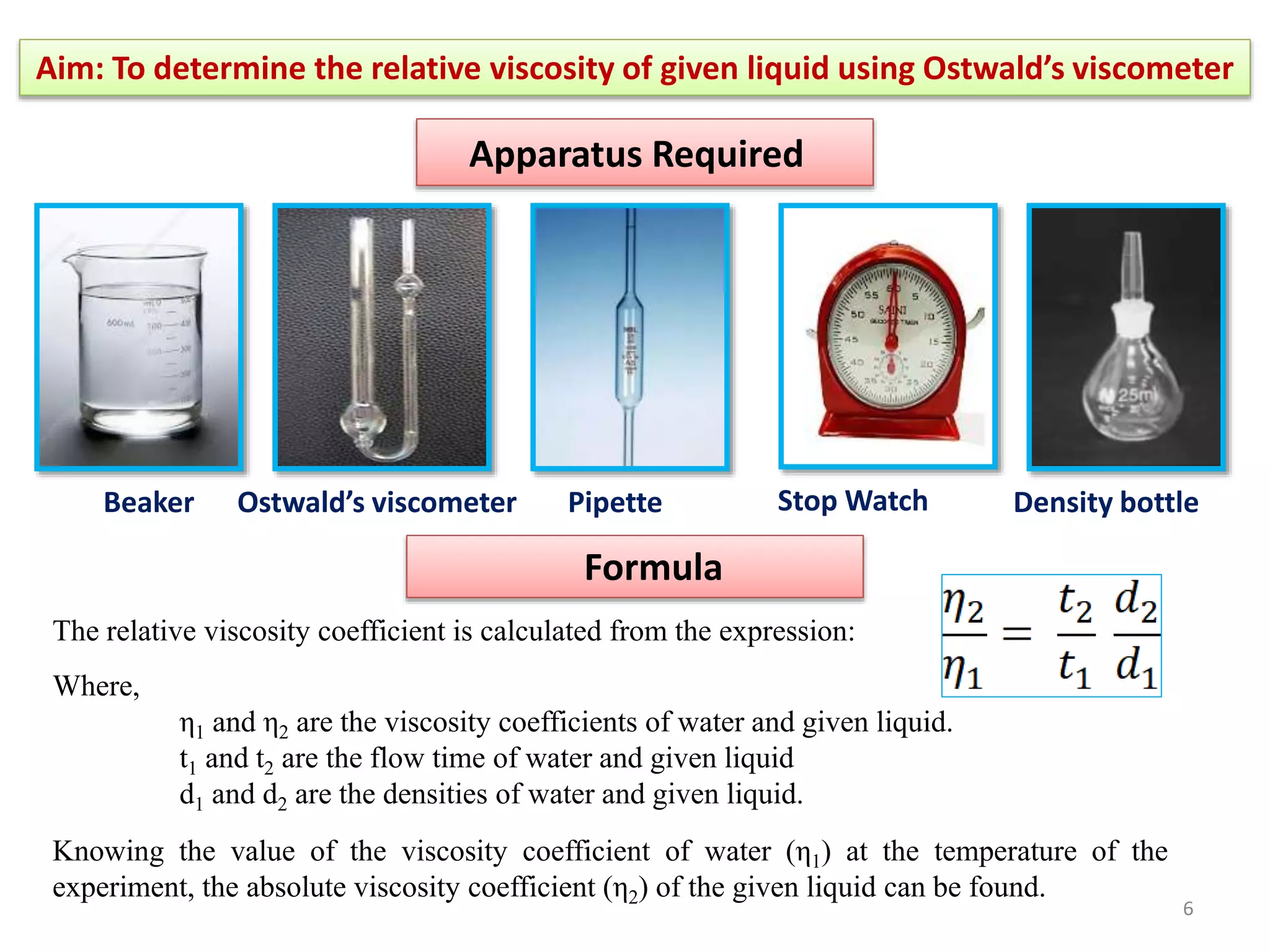
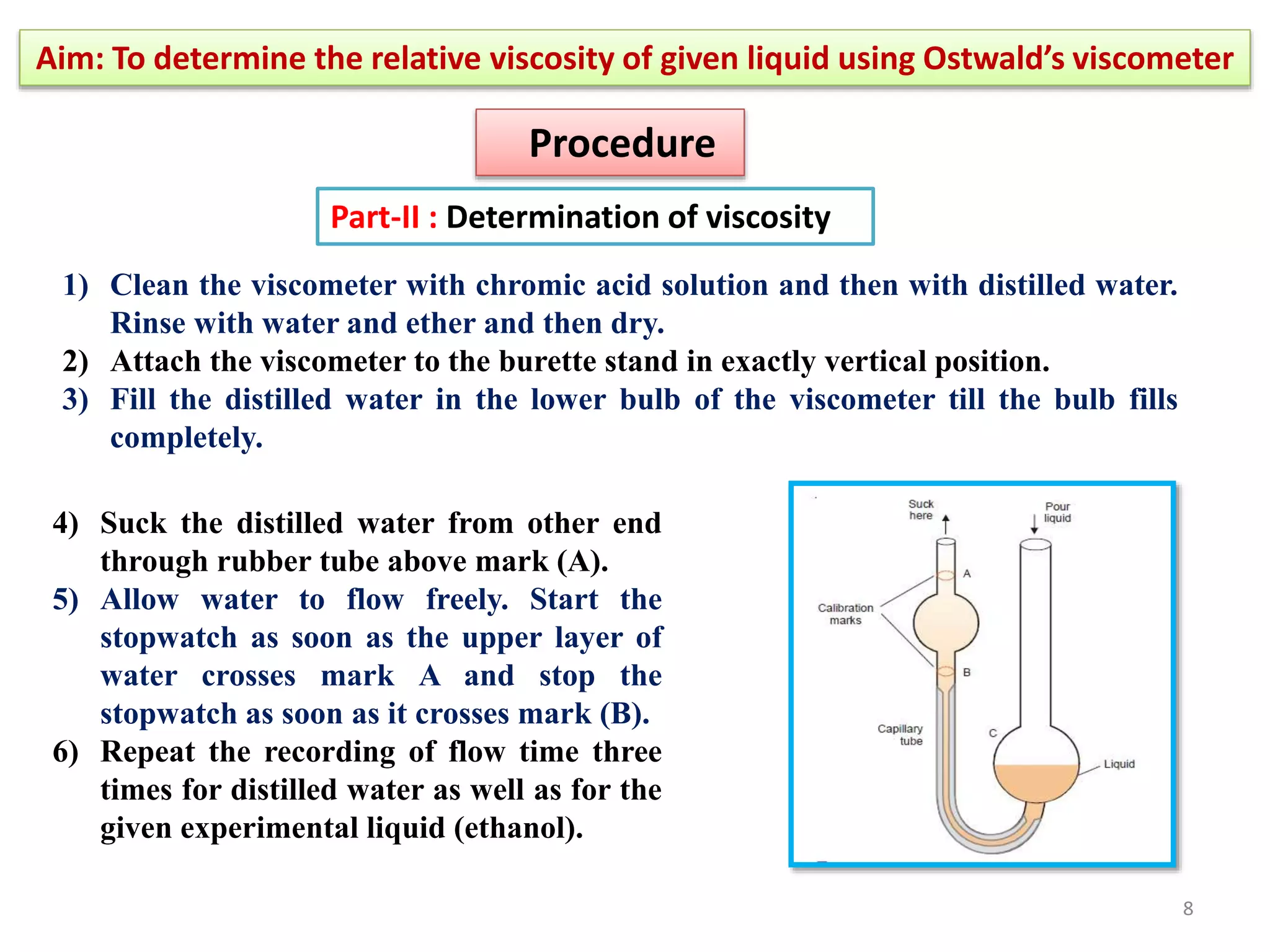
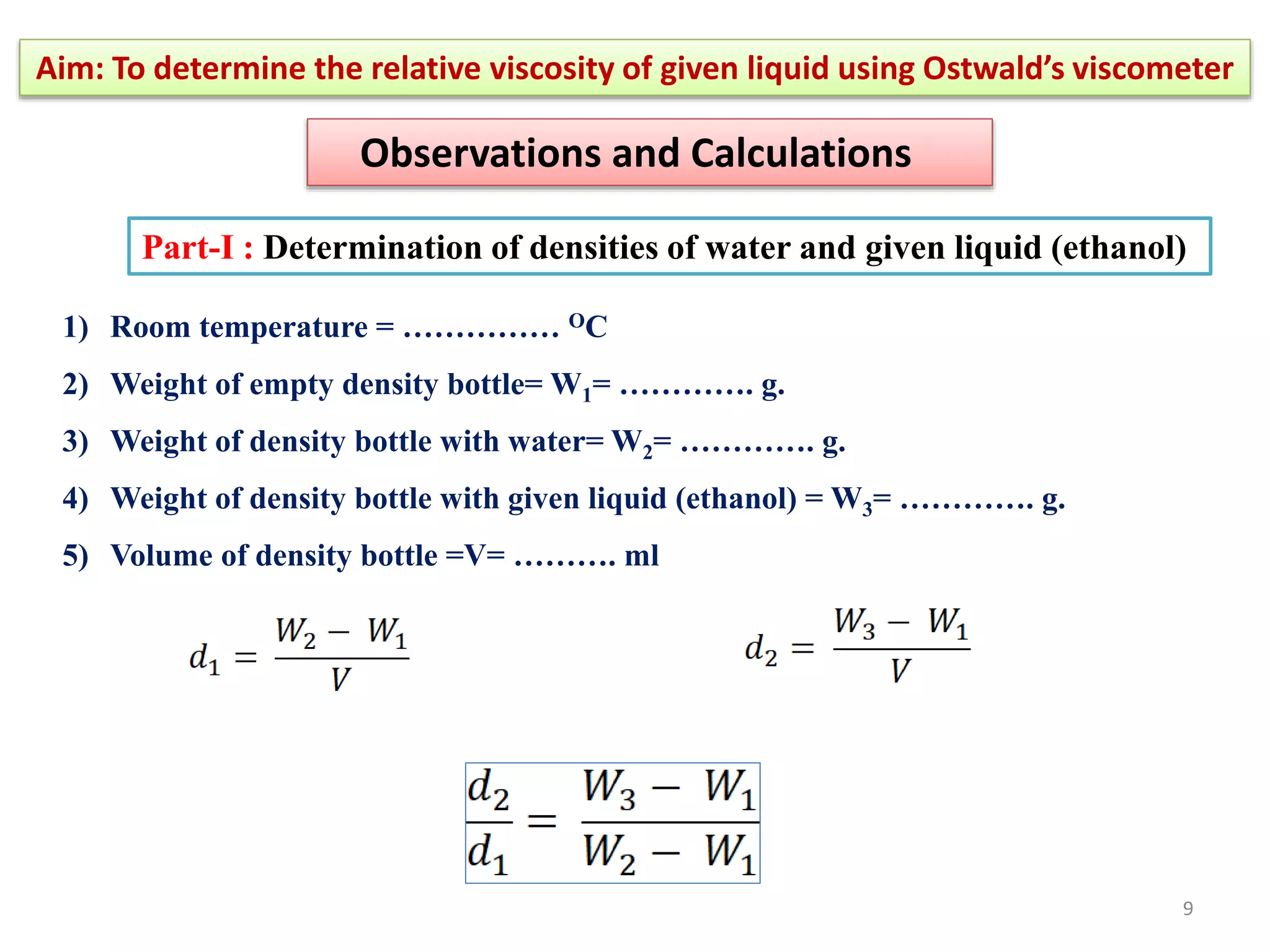
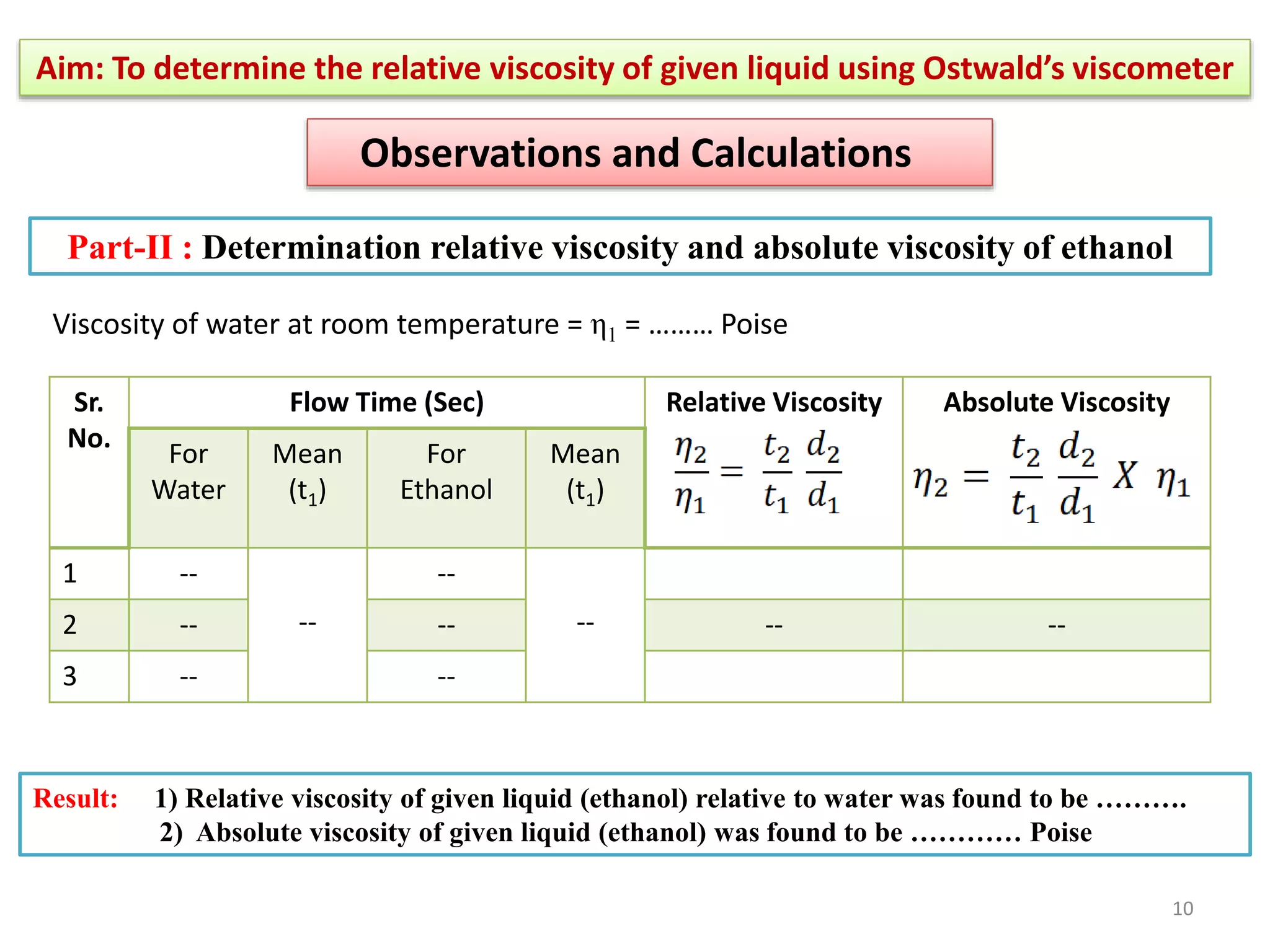
The document outlines a procedure to determine the relative viscosity of a given liquid using an Ostwald viscometer. It includes details on the apparatus, theoretical background, experimental procedures for measuring densities and viscosities, as well as methods for calculating relative and absolute viscosity. The main focus is on the relationship between flow time and viscosity, with a specific emphasis on determining the viscosity of ethanol relative to water.