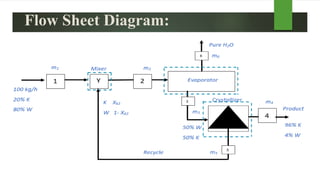
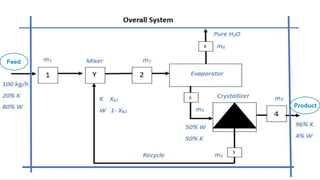
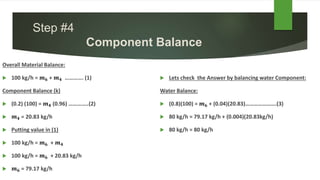
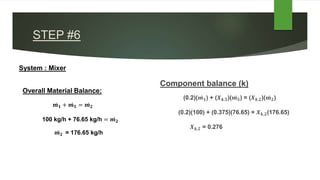
The document outlines a material balance problem involving an evaporator and a crystallizer to process a feed stream containing KNO3 and water. It details the calculations for flow rates, compositions of streams, and other parameters, ultimately leading to a component balance analysis. The results indicate the final mass fractions and flow rates for both the evaporator and crystallizer systems.