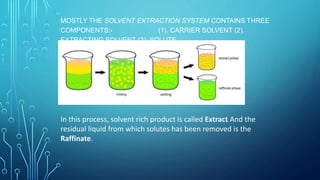
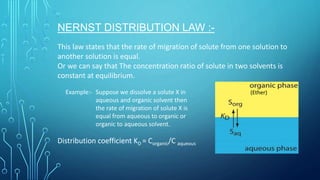
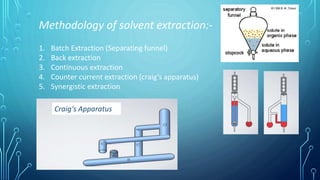
Solvent extraction is a separation process that uses immiscible solvents to transfer a solute from an aqueous solution to an organic solution. It relies on the differential solubility of compounds in two different immiscible solvents. Key aspects of solvent extraction include the Nernst distribution law, which states that the concentration ratio of a solute between the two solvents is constant at equilibrium. Solvent extraction methods include simple, multiple, continuous, and countercurrent extraction. It has various applications such as extracting uranium and plutonium from nuclear waste, separating metal ions, and recovering heat-sensitive compounds, antibiotics, and proteins.