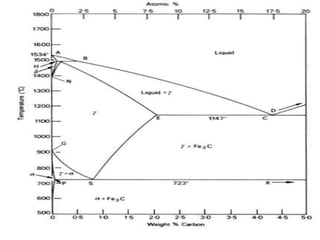
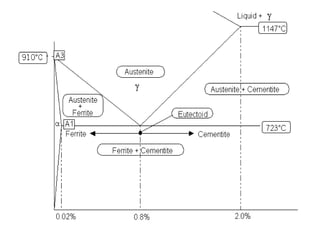
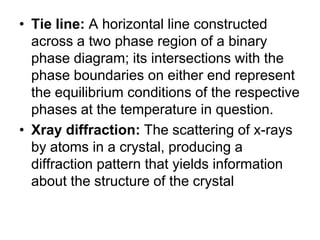
This document defines key terms related to iron-carbon phase diagrams and alloy microstructures, including different crystal structures (body-centered cubic, face-centered cubic), phases that can form in iron-carbon alloys (ferrite, austenite, cementite, pearlite, bainite, martensite), and concepts important to understanding phase diagrams (eutectic, eutectoid, solubility, lever rule). Definitions are provided for over 30 relevant materials science and metallurgy terms.