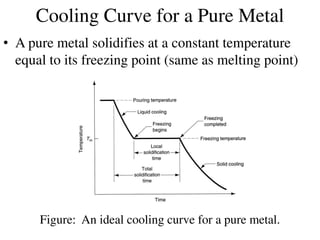
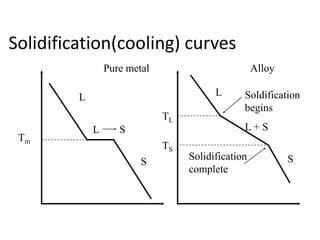
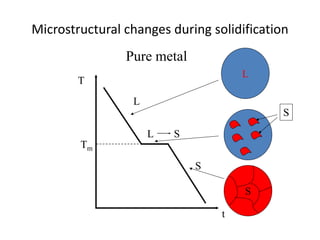
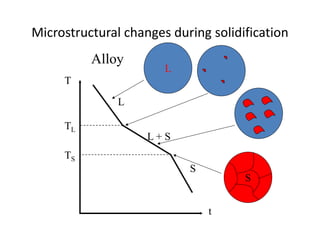
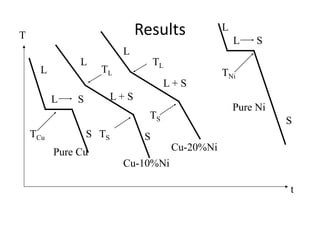
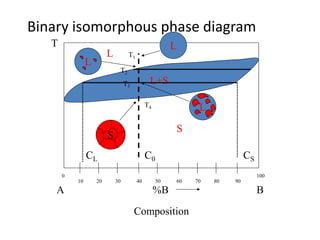
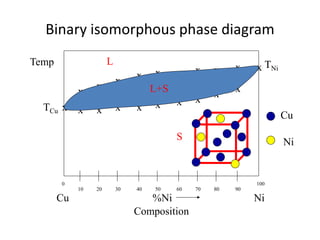
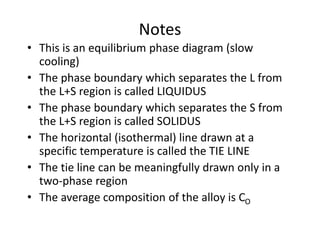
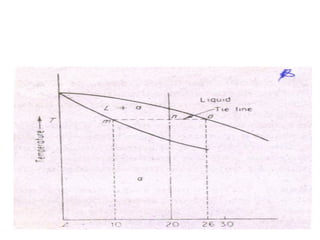
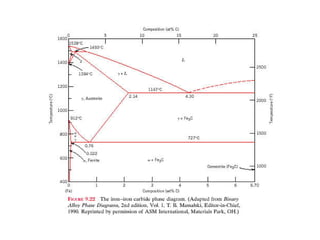
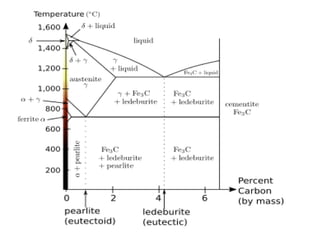
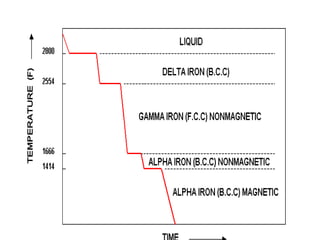
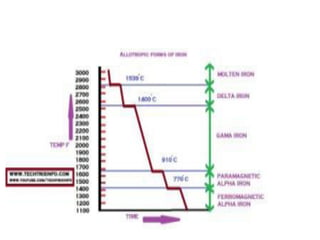
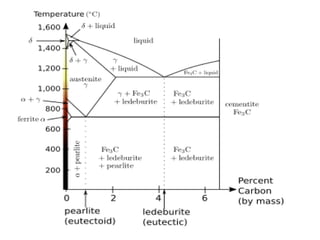
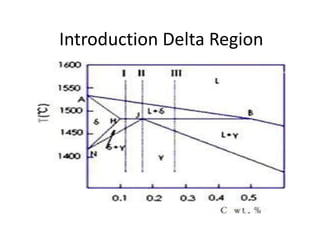
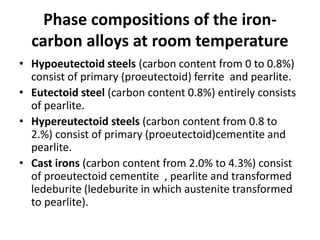
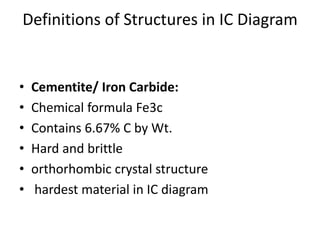
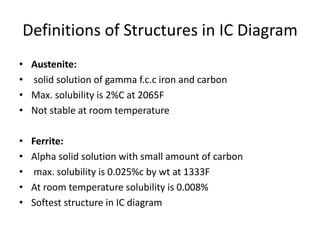
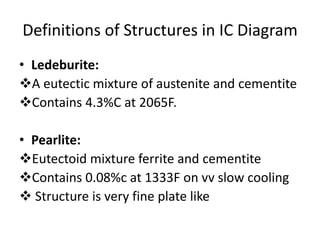
Phase diagrams graphically summarize the stable states of a substance under different conditions. The iron-carbon phase diagram shows the phases present in iron-carbon alloys at various temperatures and carbon concentrations. It indicates that iron exists in ferrite, austenite, cementite, and pearlite phases. The diagram also shows eutectic and eutectoid reactions that occur during the solidification of iron-carbon alloys. Pearlite has a lamellar structure of alternating ferrite and cementite layers.