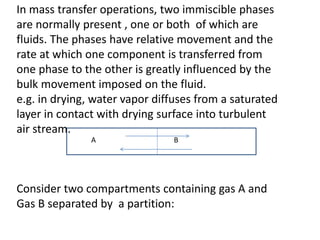
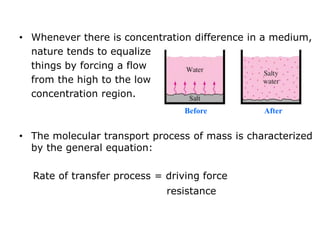
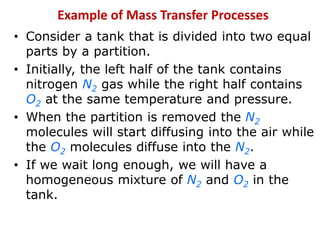
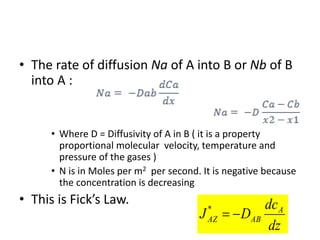
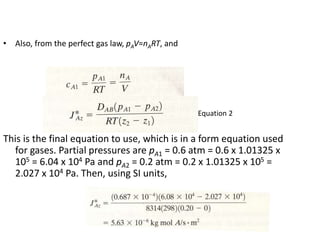
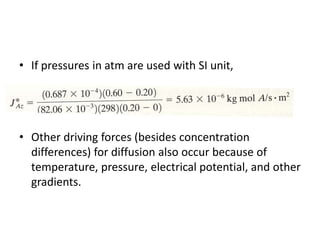
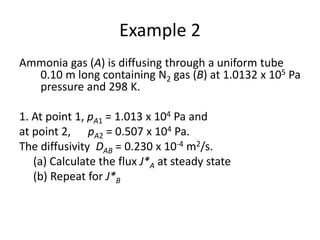
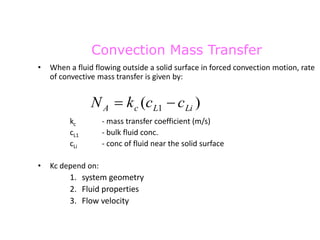
The document introduces mass transfer as a fundamental process involving the movement of mass between locations influenced by concentration gradients. It outlines various mass transfer operations, such as diffusion, distillation, and drying, which occur in different phases (gas, liquid, solid) and are characterized by driving forces like concentration, temperature, and pressure gradients. Key principles, including Fick's law of diffusion and examples of calculation in steady-state scenarios, are discussed to illustrate the mechanics of mass transfer.