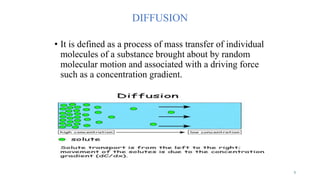
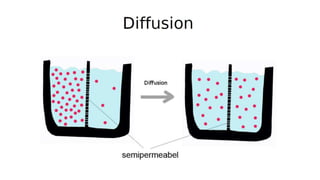
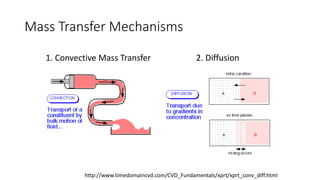
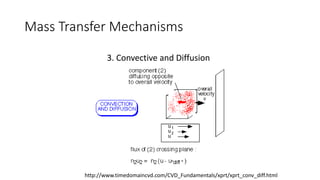
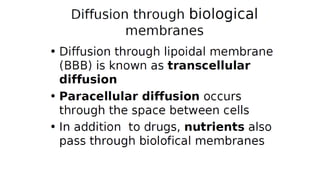
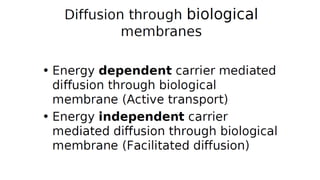
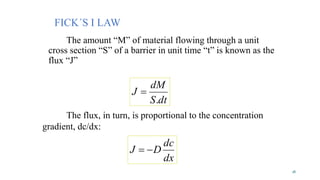
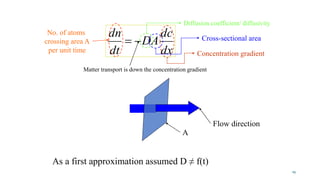
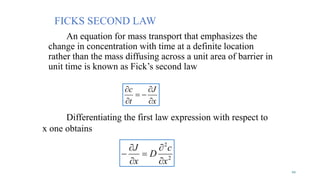
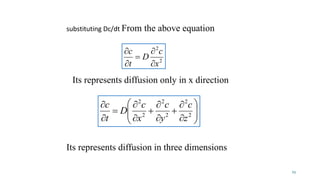
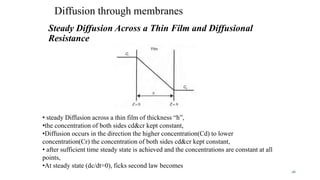
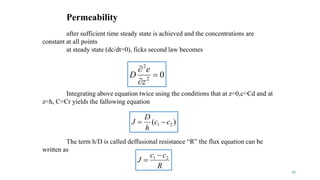
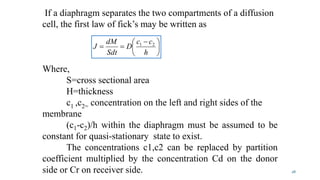
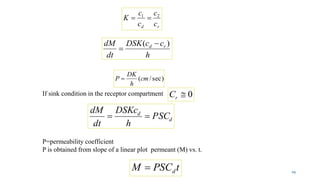
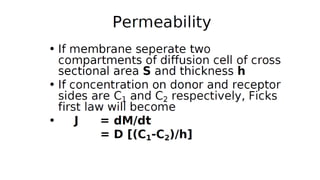
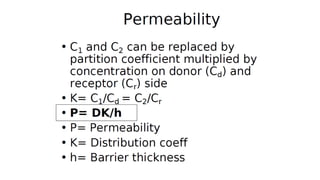
Mass transfer is the movement of mass from one location to another, such as between phases or components. It occurs through various processes like absorption, evaporation, drying, and distillation. Mass transfer involves both diffusive and convective transport mechanisms. Diffusion is the movement of molecules from a region of higher concentration to lower concentration down a concentration gradient via random molecular motion. Convection involves the bulk flow of a fluid carrying dissolved or suspended materials. Fick's first law describes the rate of diffusion, while Fick's second law describes how concentration changes over time at a given point for diffusion.