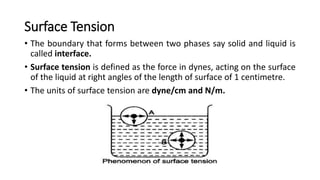
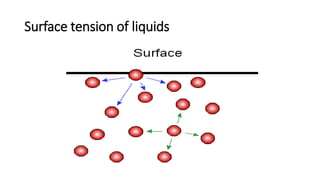
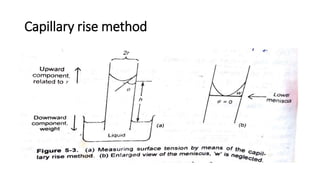
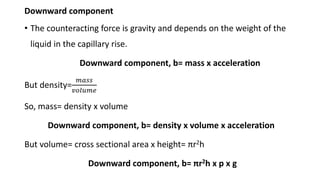
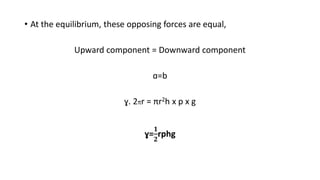
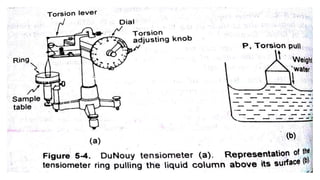
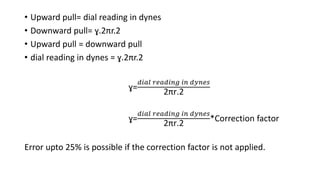
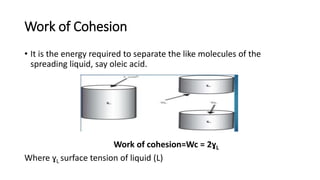
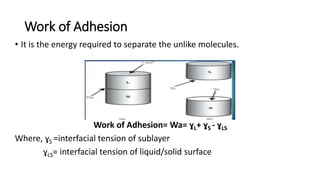
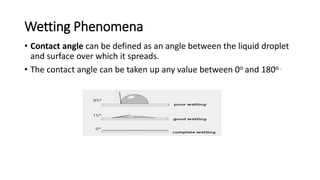
This document discusses interfacial phenomena such as surface tension, capillary rise, and wetting. It defines surface tension and explains how it is responsible for processes like droplet formation. It describes methods to determine surface and interfacial tension, including the capillary rise and Du Nouy ring methods. It also discusses concepts like surface free energy, spreading coefficients, work of cohesion/adhesion, and wetting phenomena. Wetting is important for processes like granulation, film coating, and dissolution. Surfactants can aid wetting by lowering interfacial tension and contact angles.