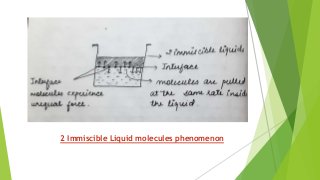
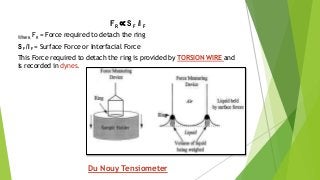
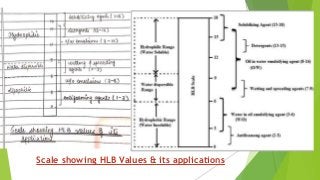
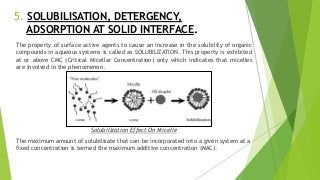
The document covers fundamental concepts of physical pharmacy relating to solubility, surface and interfacial tension, and methods for measuring these phenomena. It discusses the definitions, calculations, and instruments used in measuring surface tension, solubilization mechanics, detergency, and adsorption at solid interfaces. Additionally, it introduces the hydrophilic-lipophilic balance (HLB) scale for surfactants and their applications in emulsion formation.