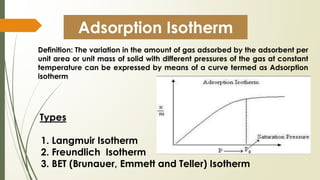
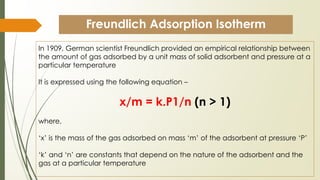
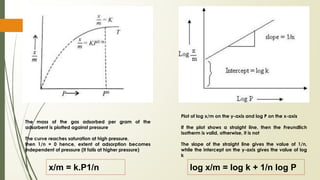
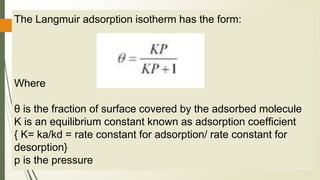
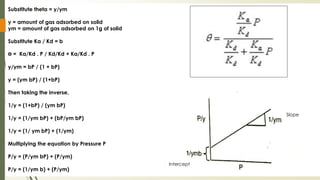
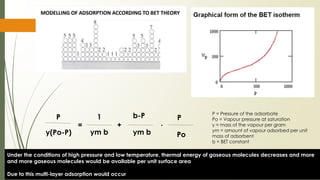
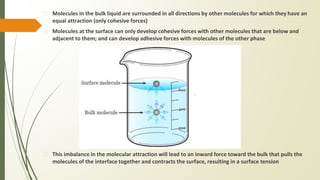
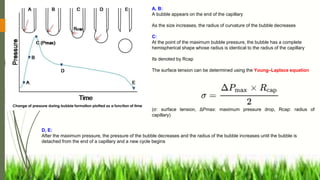
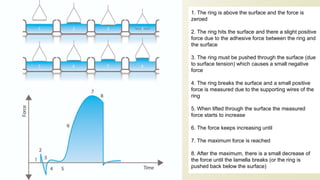
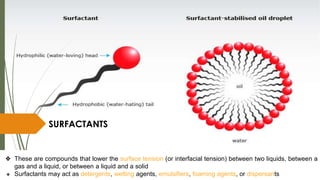
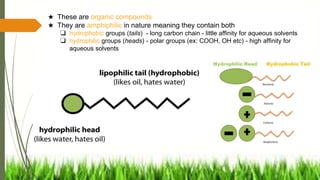
The document discusses surface and interfacial phenomena, including liquid interfaces, surface tension, interfacial tension, and adsorption at liquid interfaces. It elaborates on the measurement methods for surface and interfacial tensions, as well as the roles of surfactants in pharmaceutical applications, detailing their classifications and the hydrophilic-lipophilic balance (HLB) scale. The text emphasizes the significance of these concepts in drug formulation, stability, and solubilization technologies.











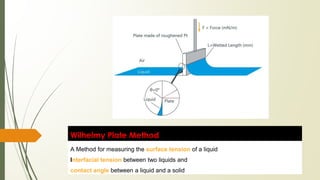
![Principle: When a vertically suspended plate touches a
liquid surface or interface, then a force F, which correlates
with the surface tension or interfacial tension 𝜸 and with
the contact angle θ according to the following equation,
acts on this plate:
L = 2 [ w + D ] where, w is the plate width and D is the plate thickness
𝜸 = F / L cos ø](https://image.slidesharecdn.com/surfaceandinterfacialphenomenon-220126170231/85/Surface-and-interfacial-phenomenon-18-320.jpg)




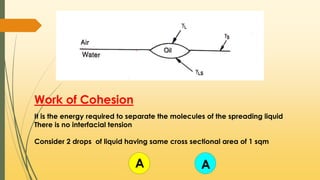
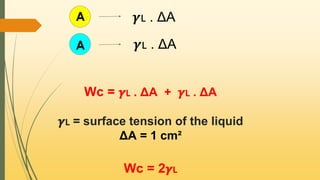
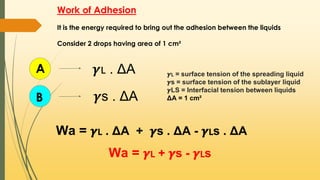
![When the adhesive forces are stronger than the cohesive forces,
then the spreading occurs
S = Wa - Wс
S = 𝜸🇱 + 𝜸s - 𝜸🇱s - 2𝜸🇱
S = 𝜸s - 𝜸🇱s - 𝜸🇱
s = 𝜸s - [𝜸🇱 + 𝜸🇱s]
If 𝜸s > [𝜸🇱 + 𝜸🇱s] then S is positive indicates Spreading
If 𝜸s < [𝜸🇱 + 𝜸🇱s] then S is negative indicates no Spreading](https://image.slidesharecdn.com/surfaceandinterfacialphenomenon-220126170231/85/Surface-and-interfacial-phenomenon-28-320.jpg)

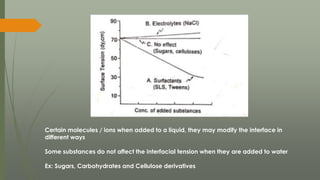
![Adsorption at Liquid Interfaces
Positive Adsorption
[Partitioned in the favour of the
interface]
When the added molecules
move on their own accord to
interface
Surface free energy and Surface
tension get decreased
Ex: Surfactants: Sodium lauryl
sulphate, Tweens and
Triethanolamine
Negative Adsorption
[Partitioned in the favour of
the bulk]
When the added molecules
prefer to remain in the bulk
of solution
Enhances surface tension
Ex: Inorganic electrolytes:
sodium chloride](https://image.slidesharecdn.com/surfaceandinterfacialphenomenon-220126170231/85/Surface-and-interfacial-phenomenon-30-320.jpg)






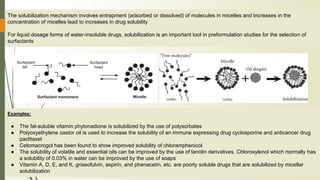



![Types of Adsorption
Physical Adsorption
Example: Adsorption of gases on charcoal
Force: Van der Waals weak intermolecular
interaction
Nature of gas: Easily liquefiable gases are
readily adsorbed
Effect of Pressure: P directly proportional to
the extent of adsorption
Reversible nature: Decrease in Pressure
causes Desorption (reversible)
Effect of Temperature: Favoured at low
temperature [10-40 KJ/mol]
Thickness of adsorbed layer: unimolecular at
low pressure and multi-molecular at higher
pressure
Chemical Adsorption
Example: Adsorption of oxygen on silver or
gold
Force: Chemical bonds
Nature of gas: Highly specific
Effect of Pressure: P directly proportional to
the extent of adsorption until the surface gets
saturated, after that P has no effect
Reversible nature: Decrease in pressure does
not cause desorption (irreversible)
Effect of Temperature: Favoured at high
temperature [ > 40 KJ/mol]
Thickness of adsorbed layer: unimolecular](https://image.slidesharecdn.com/surfaceandinterfacialphenomenon-220126170231/85/Surface-and-interfacial-phenomenon-50-320.jpg)