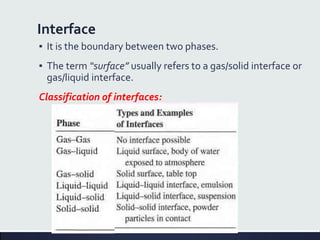
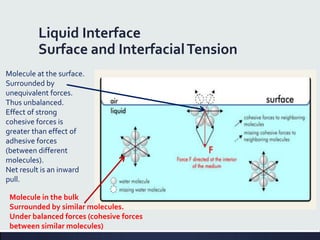
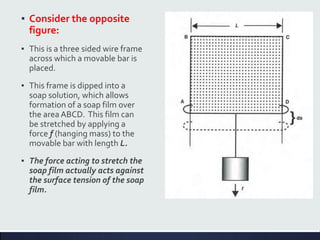
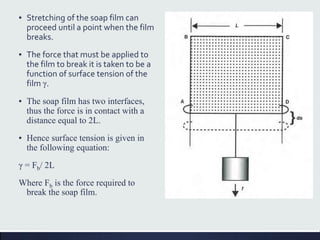
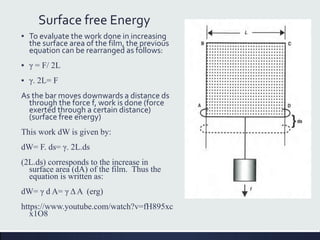
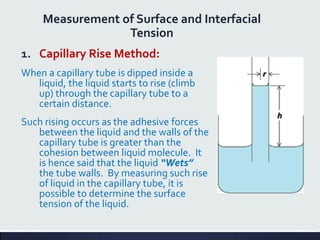
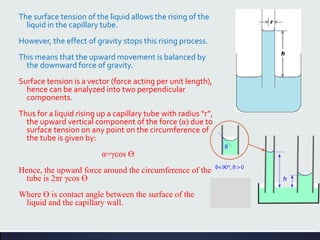
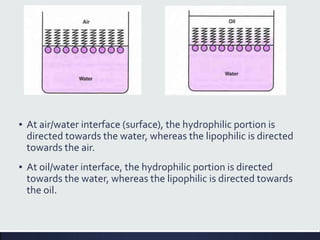
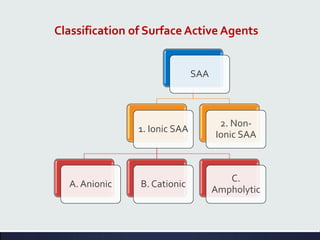
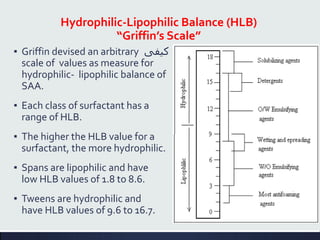
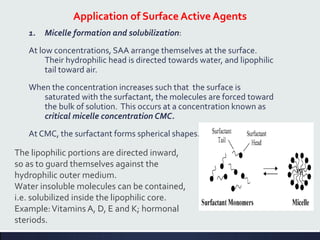
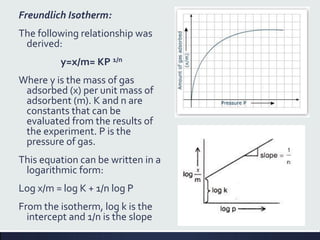
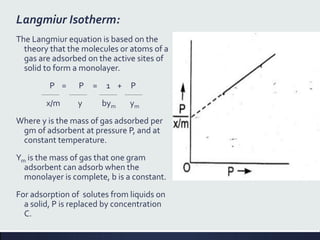
This document discusses interfaces and interfacial phenomena. It defines an interface as the boundary between two phases and classifies interfaces as either liquid, solid, or gas interfaces. It then discusses concepts such as surface tension, interfacial tension, and how they arise from unbalanced cohesive and adhesive forces at interfaces. Various methods for measuring surface and interfacial tensions are also presented, including the capillary rise method and DuNouy tensiometer. The document concludes by covering topics such as surface active agents/surfactants, their classification and applications, as well as adsorption phenomena at solid interfaces.