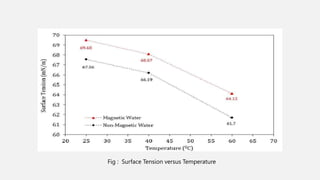
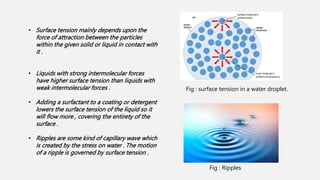
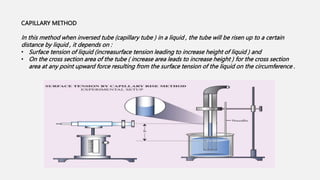
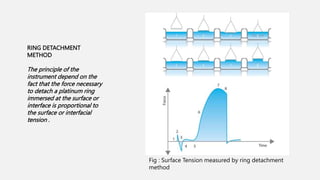
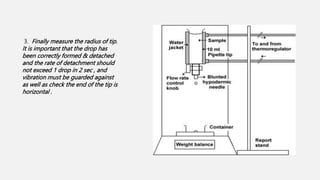
This document discusses methods for determining surface tension, including the drop weight method. It begins by defining surface tension and explaining that it is caused by cohesive forces between liquid molecules being stronger than adhesive forces between liquid and air molecules. It then describes several methods to measure surface tension, including the capillary method, ring detachment method, and various drop methods. The drop weight method specifically measures surface tension based on the weight and radius of drops detached from a tube under constant conditions. Factors affecting surface tension and applications are also discussed.

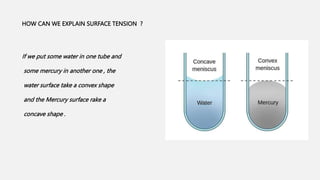
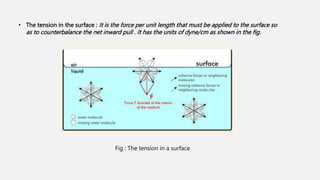
![• At liquid – air interfaces , surface tension results from the greater attraction of liquid molecules to each
other ( due to cohesion ) than to molecules in the air ( due to adhesion ) .
• Surface tension has the dimension of force per unit length or energy per unit area . The two are
equivalent , but when referring to energy per unit area , it is common to use the term surface energy ,
which is more general term in the sense that it applies also to solids .
• In material science, surface tension is used for either surface stress or surface free energy.
• SI unit of Surface Tension is (N/m) or (J/m²)
• Its dimension is [M⁰ L¹ T ‐ ² ]
• Angle of contact : The angle measured from the side of the liquid , between the tangent to the solid
surface inside the liquid and tangent to the free liquid surface at the point of contact between solid
and liquid surfaces .](https://image.slidesharecdn.com/surfacetension-230414151410-4fdc5017/85/Surface-tension-pptx-3-320.jpg)