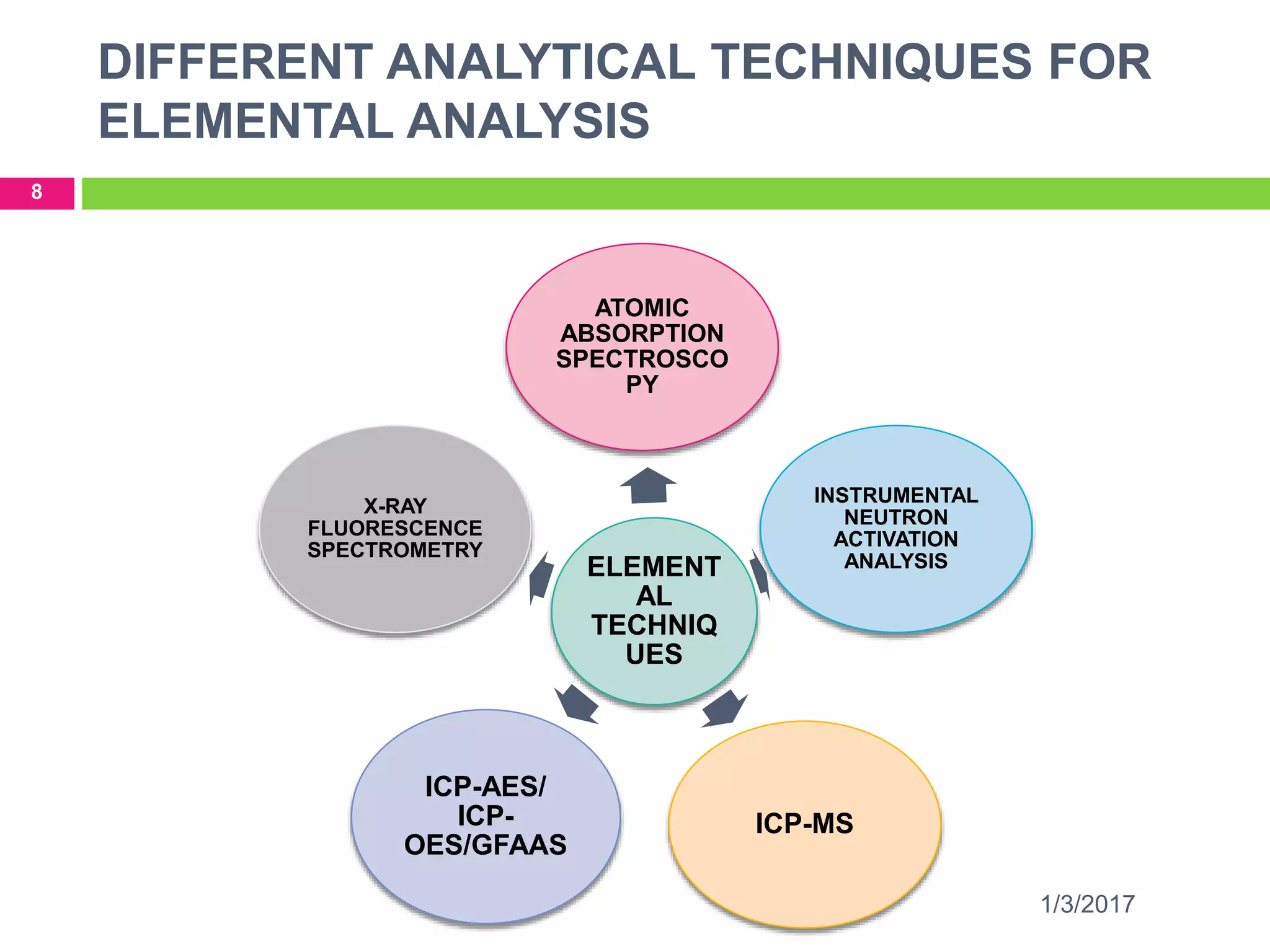
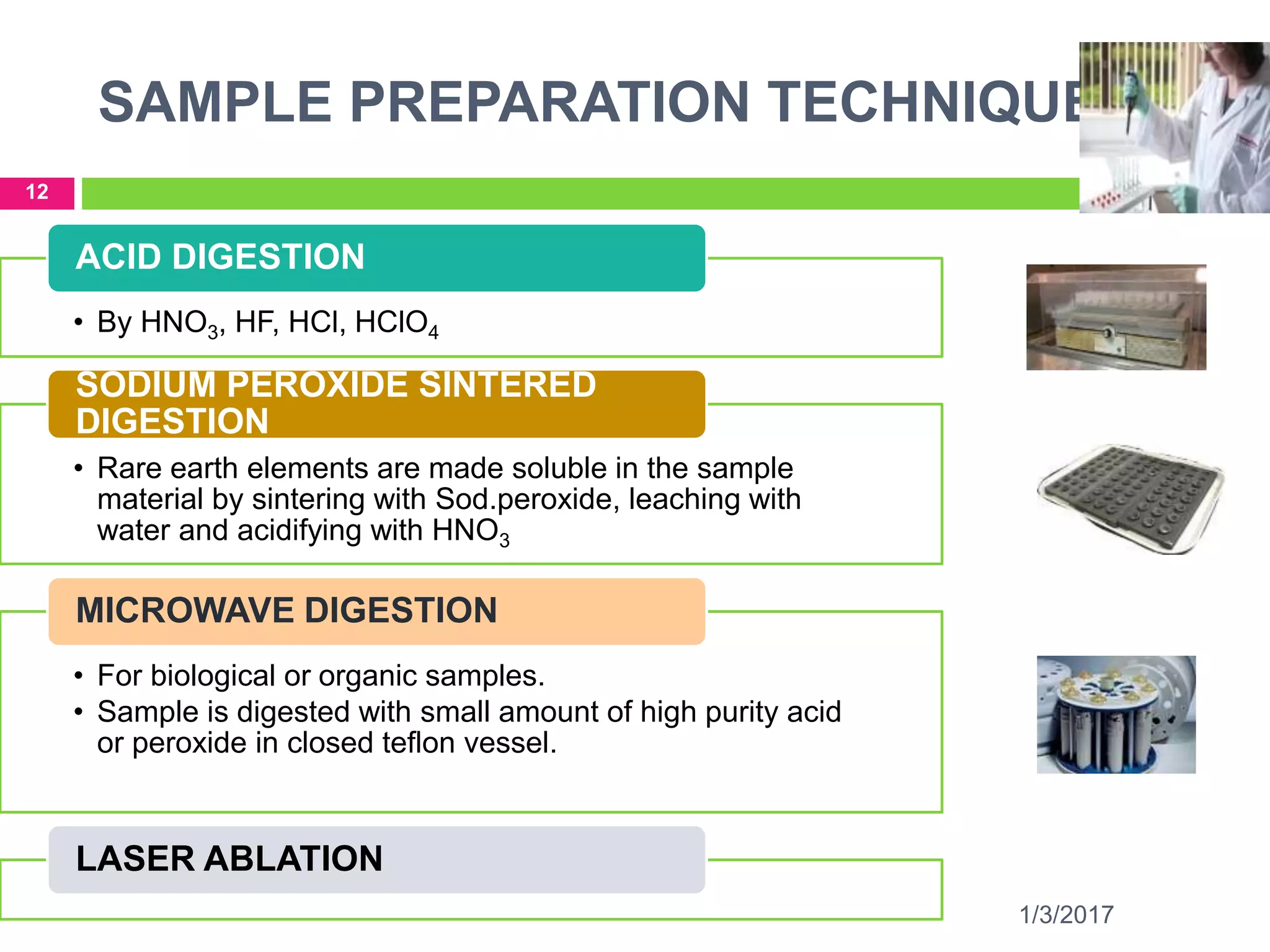
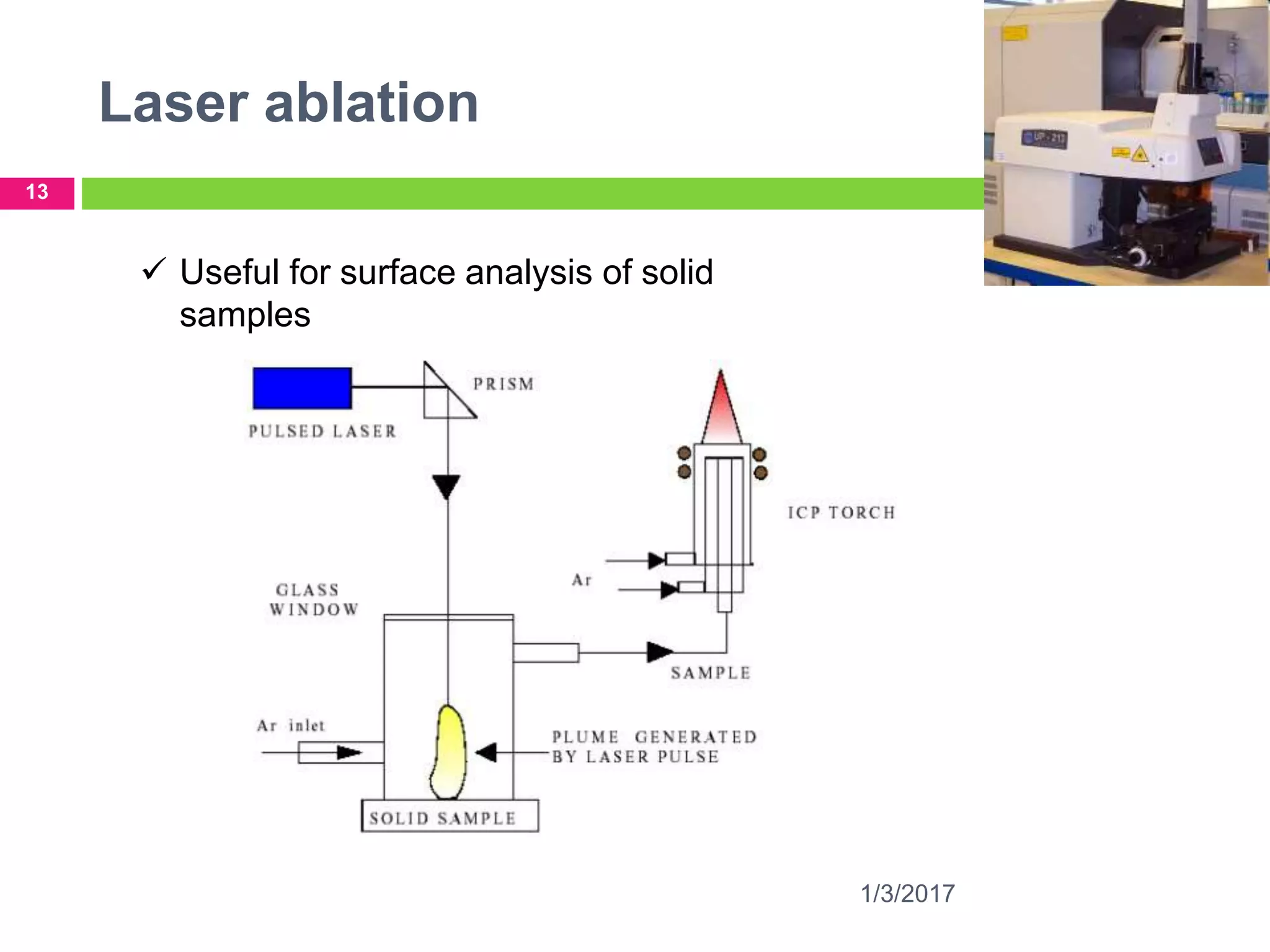
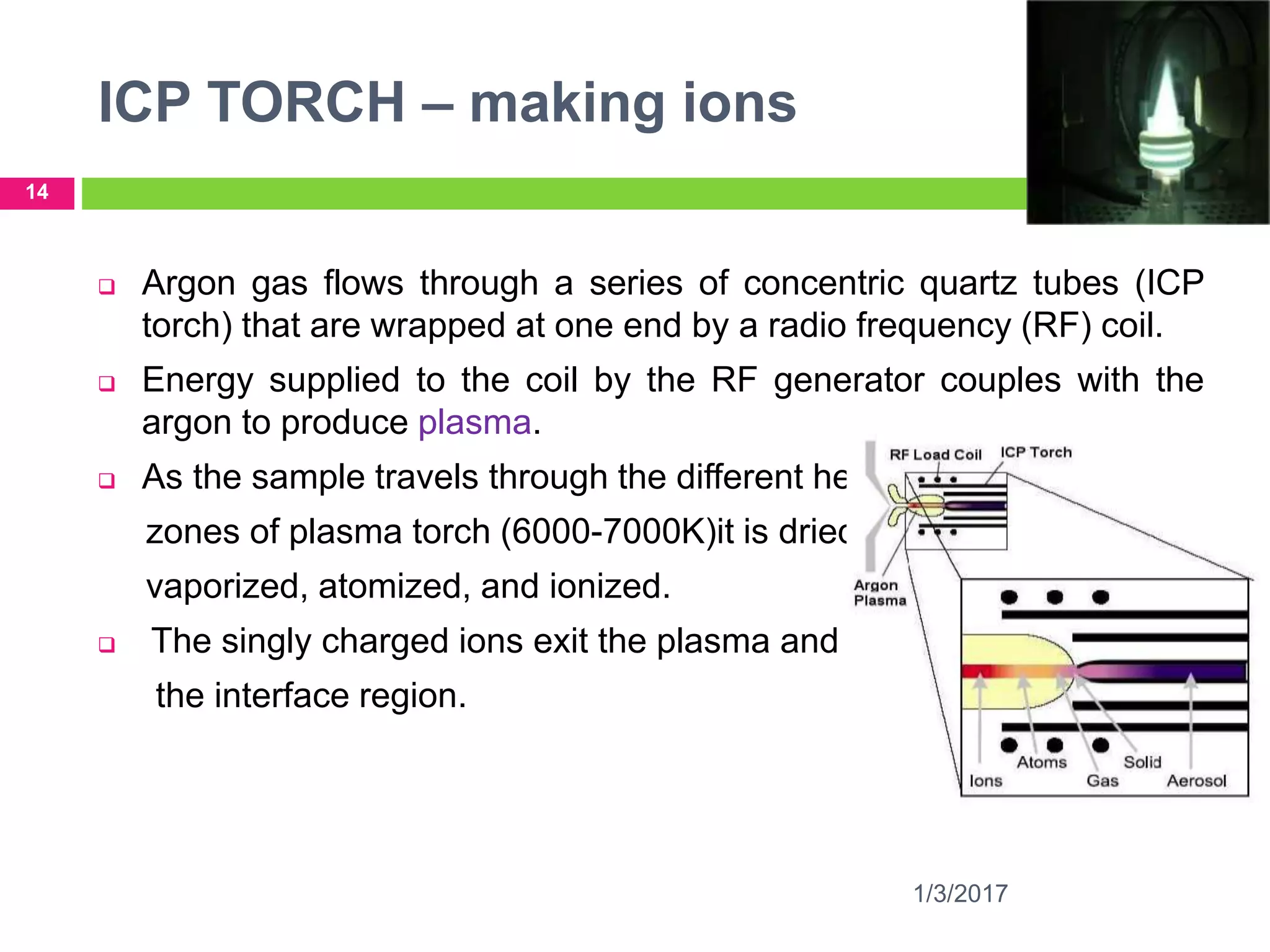
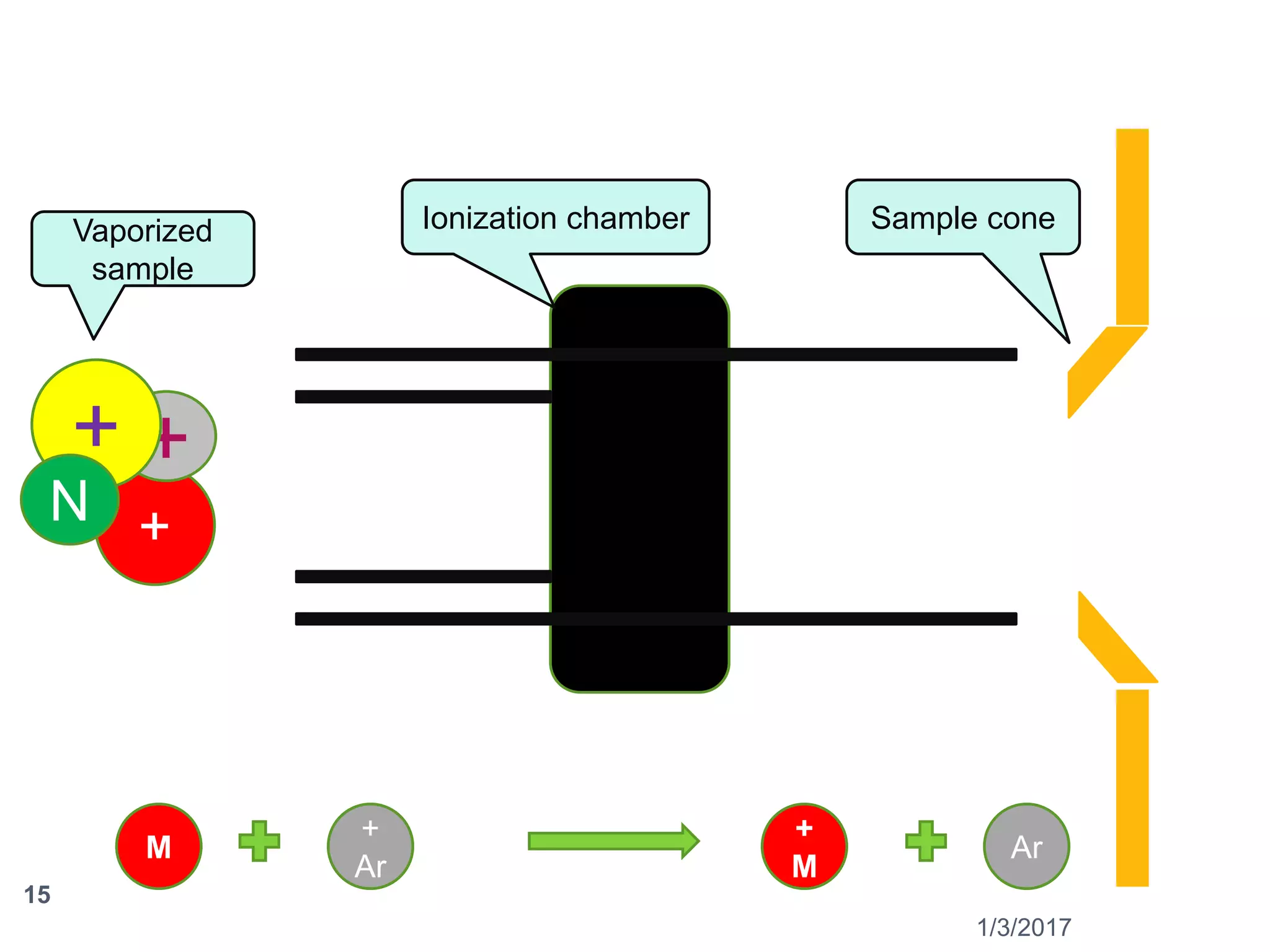
The document discusses the importance of monitoring elemental impurities in pharmaceuticals, detailing regulatory compliance and specific guidelines for acceptable limits of various trace metals using ICP-MS (Inductively Coupled Plasma Mass Spectrometry). It outlines the principles of ICP-MS, including sample preparation and ionization techniques, and compares different analytical methods, highlighting advantages and limitations. The conclusion emphasizes ICP-MS as a highly effective technique for achieving low detection limits and high productivity in elemental analysis.



















![REFERENCES
1/3/2017
36
[1] V. Balaram ; Recent advances in the determination of elemental
impurities in pharmaceuticals – Status, challenges and moving
frontiers ; Trends in Analytical Chemistry.
[2] R.N. Rao, M.K. Talluri, An overview of recent applications of
inductively coupled plasma-mass spectrometry (ICP-MS) in
determination of inorganic impurities in drugs and pharmaceuticals,
J. Pharm. Biomed. Anal. 43 (2007) 1-13.
[3] ICH, Guideline for Elemental Impurities Q3D, in: International
Council for Harmonisation, IFPMA, Geneva, Switzerland, 2014.
[4] Skoog, D. A., Holler, F. J., & Crouch, S. R.(2007).Principles of
Instrumental Analysis.
[5] Available from:
http://www.perkinelmer.com/icpmsthirtminuteguide.pdf](https://image.slidesharecdn.com/pa2016106-170103080942/75/Elemental-analysis-Trace-metals-by-ICP-MS-36-2048.jpg)