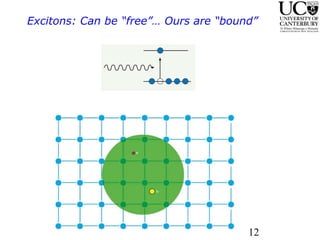
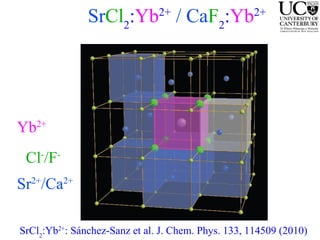
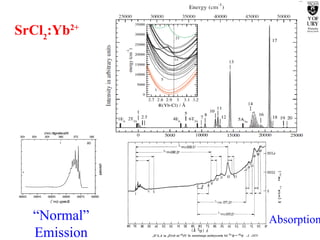
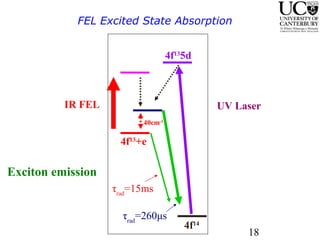
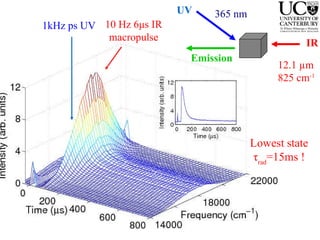
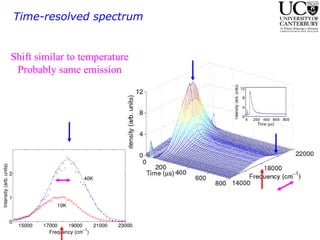
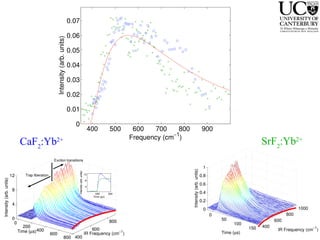
1. The document studies excitons and traps in rare-earth materials like Yb2+ doped CaF2 and SrF2 using a free-electron laser (FEL) combined with ultraviolet excitation.
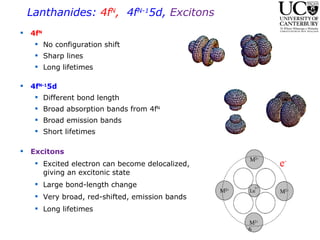
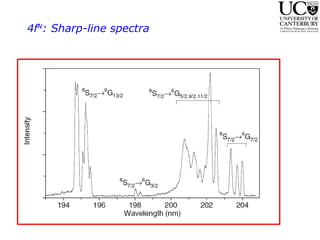
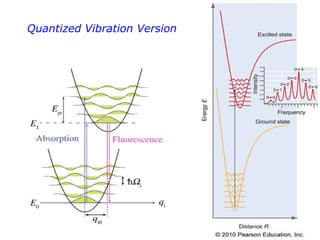
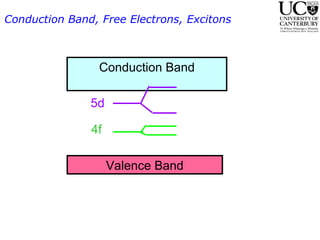
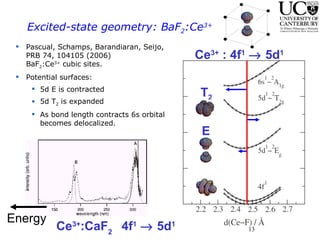
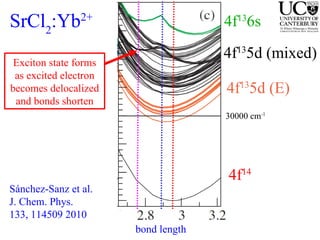
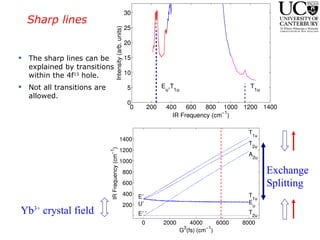
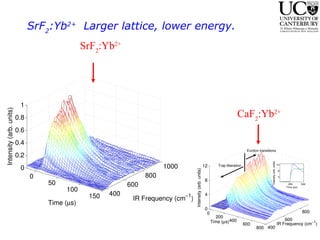
2. Excitation creates an excitonic state where the excited electron becomes delocalized, leading to bond length changes and broad, red-shifted emission bands.
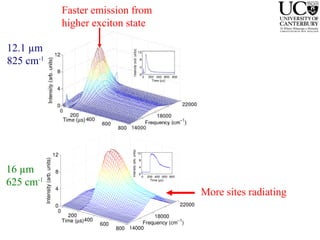
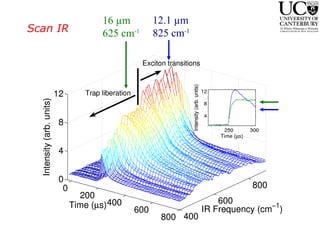
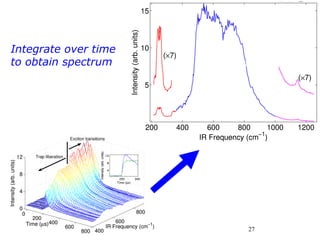
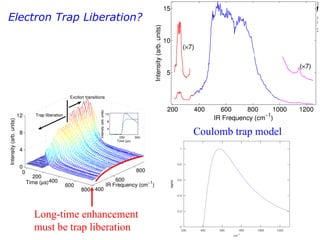
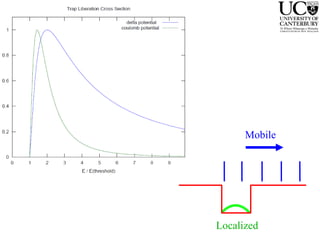
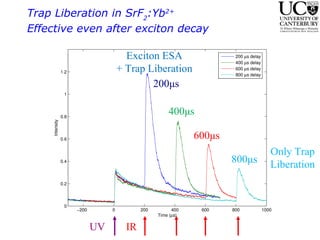
3. FEL excitation of the excitonic state is used to probe trap liberation, showing enhanced emission even after exciton decay, relevant to applications like phosphors.