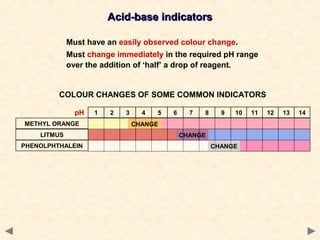
- Many acid-base indicators are weak acids that partially dissociate in solution, existing in both ionized and un-ionized forms which have different colors. In acidic solution, the equilibrium favors the un-ionized form which is typically red. In alkaline solution, the equilibrium favors the ionized blue form.
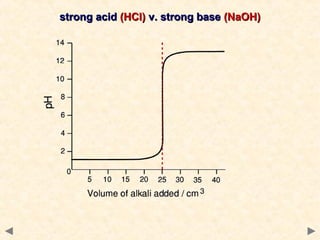
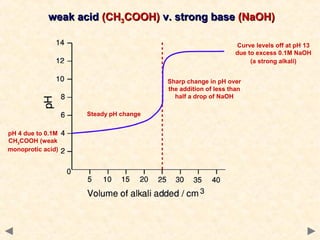
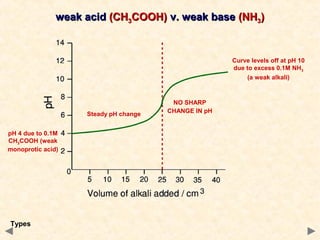
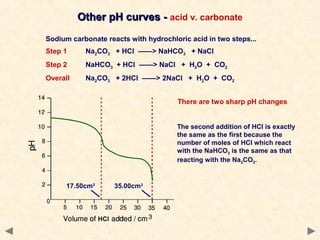
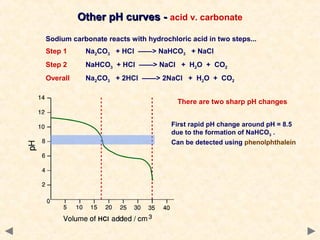
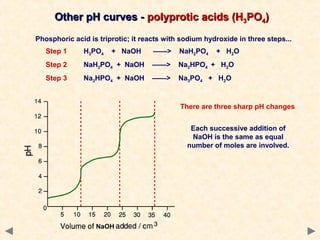
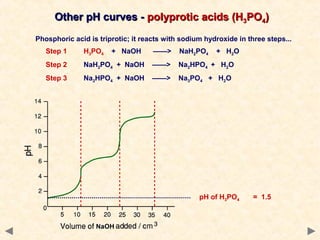
- Titration curves show characteristic shapes depending on whether the acid and base are strong or weak. A strong acid-strong base titration has an initial flat region followed by a sharp pH change at the equivalence point. A weak acid-strong base titration has a gradual pH change throughout.
- The suitable indicator for a titration must change color within the sharp pH change region near the


![Acid-base indicators
General
Many indicators are weak acids and partially dissociate in aqueous solution
HIn(aq)
H+(aq) + In¯(aq)
The un-ionised form (HIn) is a different colour to the anionic form (In¯).
Apply Le Chatelier’s Principle to predict any colour change
In acid
- increase of [H+]
- equilibrium moves to the left to give red undissociated form
In alkali - increase of [OH¯]
- OH¯ ions remove H+ ions to form water;
H+(aq) + OH¯(aq)
- equilibrium will move to the right to produce a blue colour
H2O(l)](https://image.slidesharecdn.com/indicators-131202093352-phpapp02/85/Indicators-3-320.jpg)
![Acid-base indicators
General
Many indicators are weak acids and partially dissociate in aqueous solution
HIn(aq)
H+(aq) + In¯(aq)
The un-ionised form (HIn) is a different colour to the anionic form (In¯).
Apply Le Chatelier’s Principle to predict any colour change
In acid
- increase of [H+]
- equilibrium moves to the left to give red undissociated form
In alkali - increase of [OH¯]
- OH¯ ions remove H+ ions to form water;
H+(aq) + OH¯(aq)
- equilibrium will move to the right to produce a blue colour
Summary
In acidic solution
HIn(aq)
H+(aq) + In¯(aq)
In alkaline solution
H2O(l)](https://image.slidesharecdn.com/indicators-131202093352-phpapp02/85/Indicators-4-320.jpg)