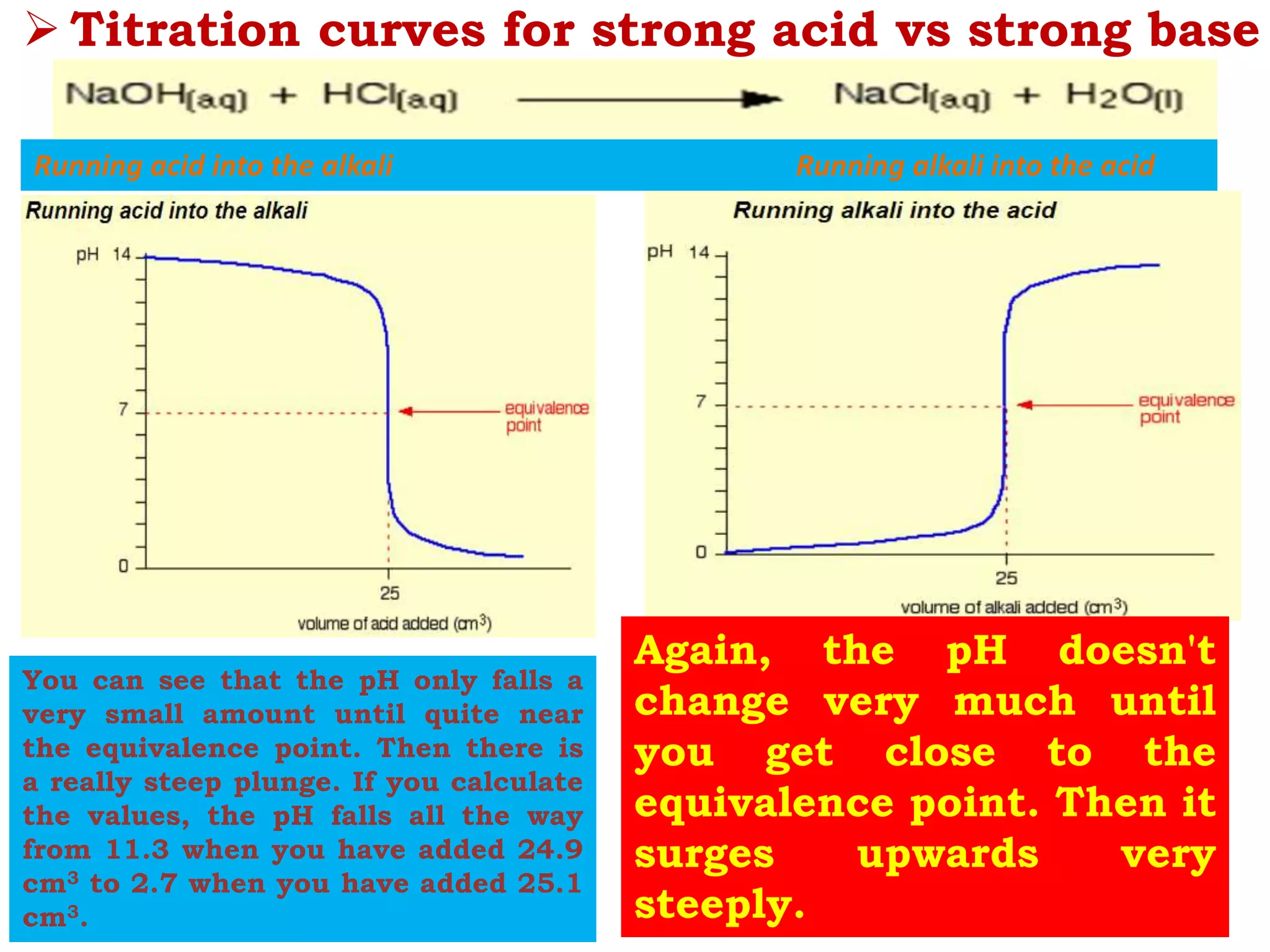
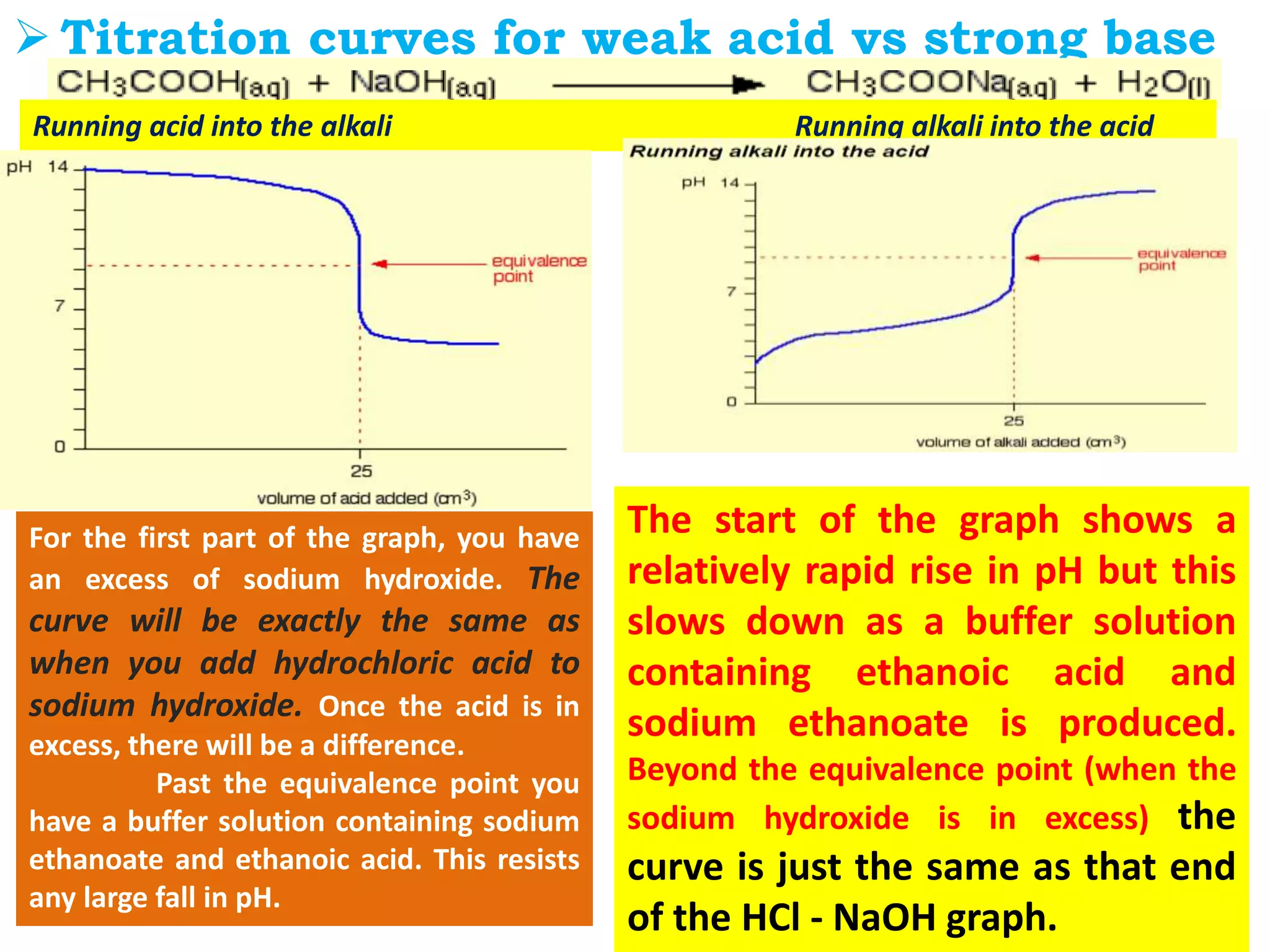
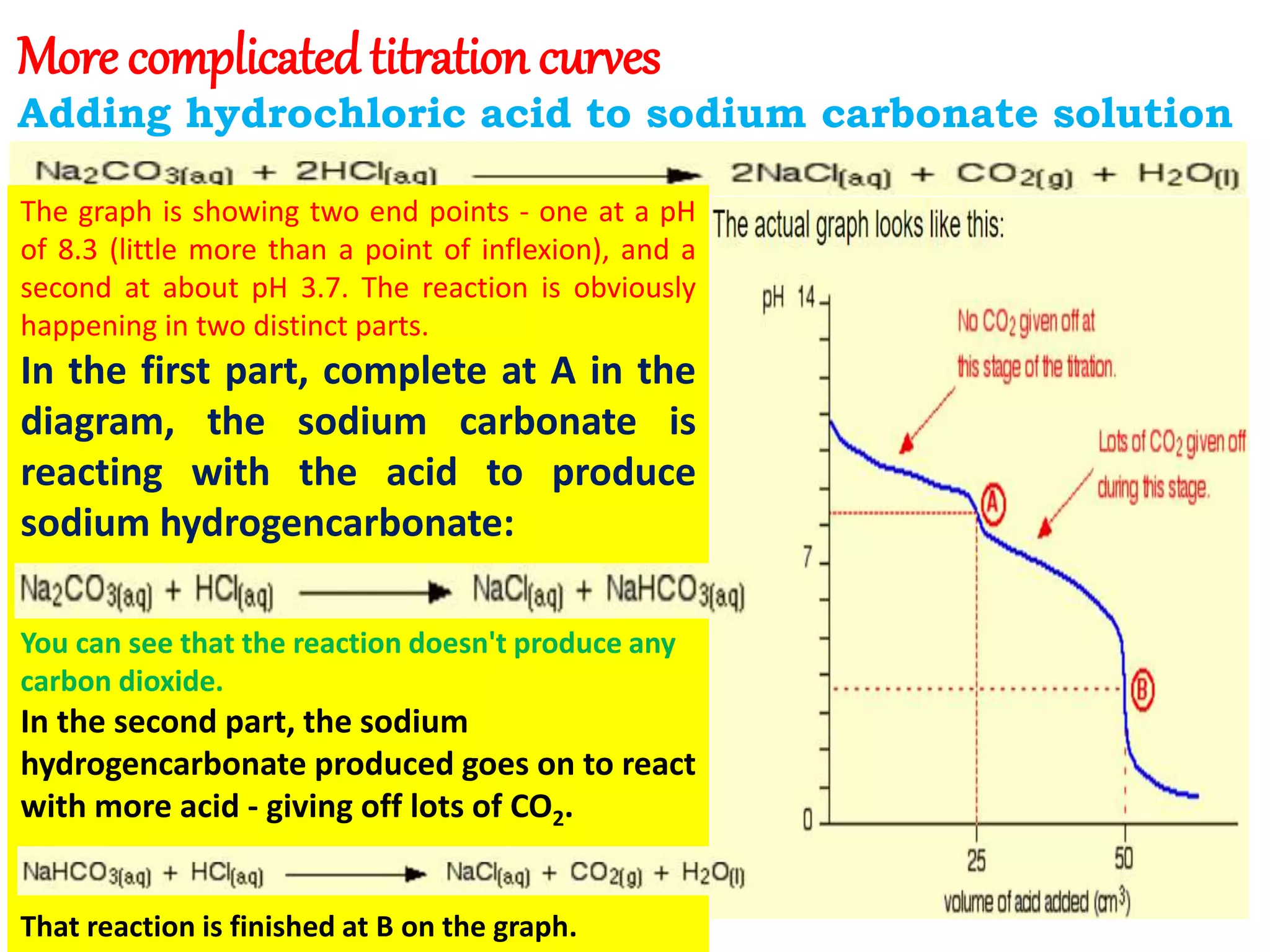
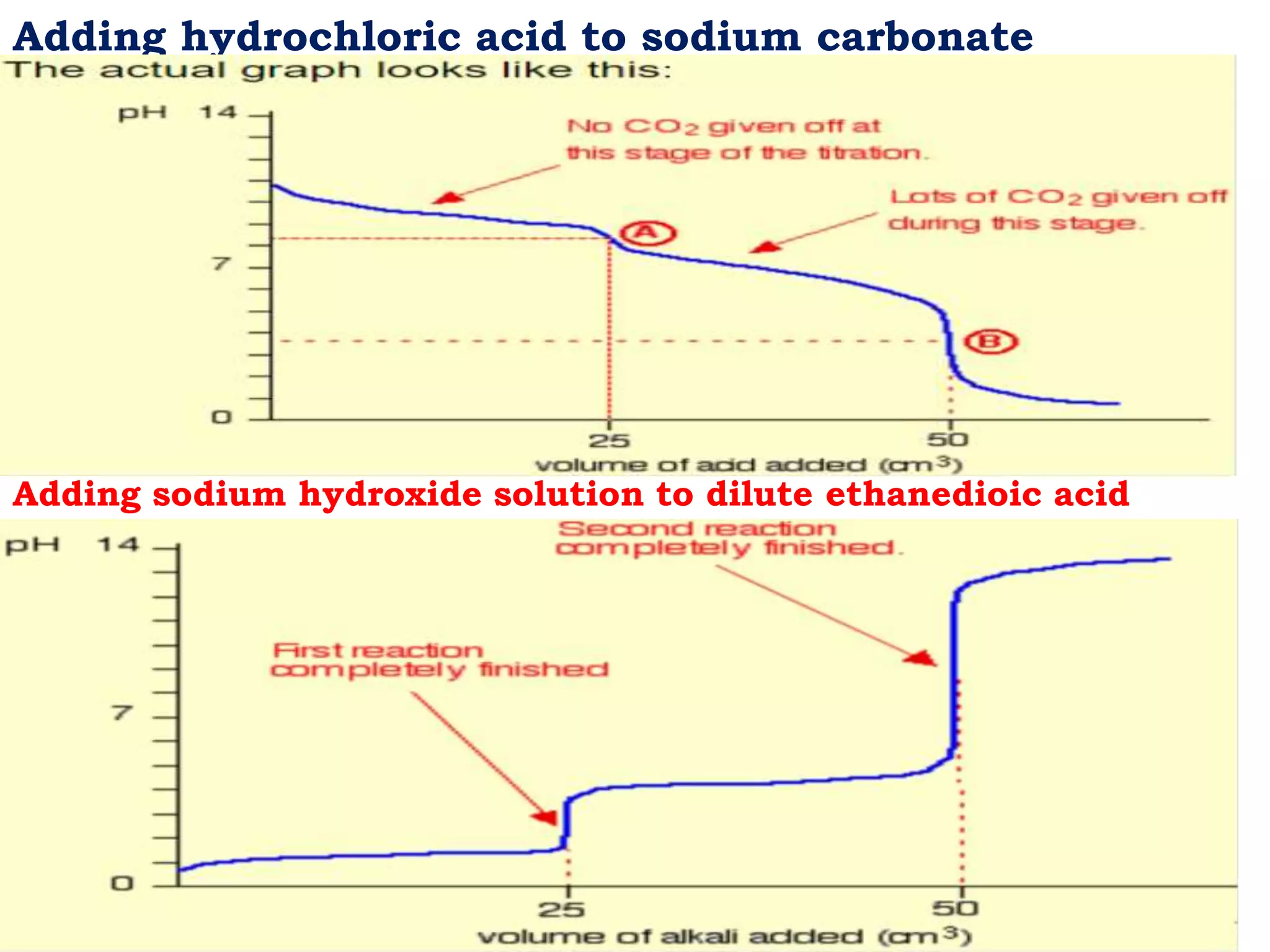
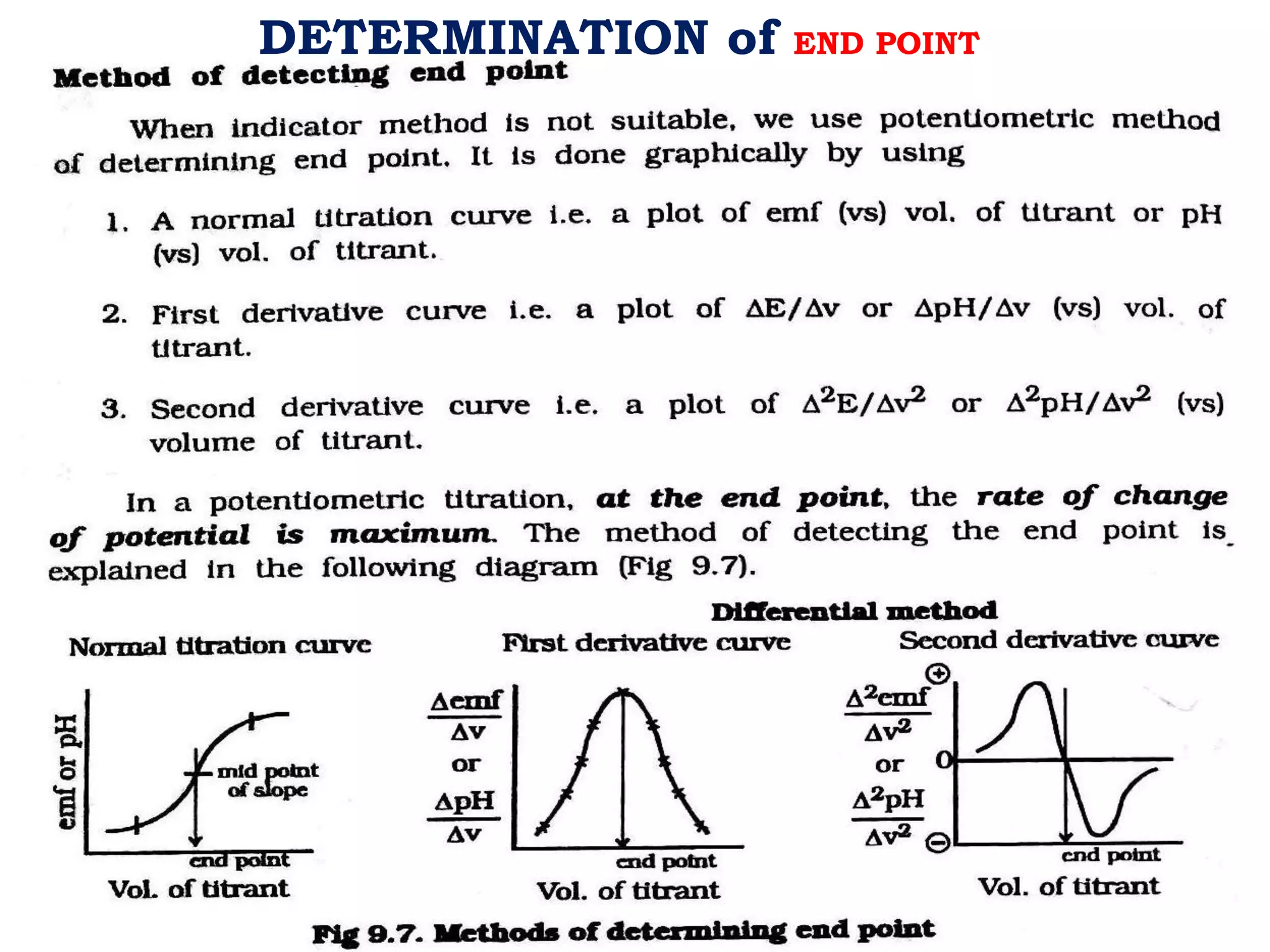
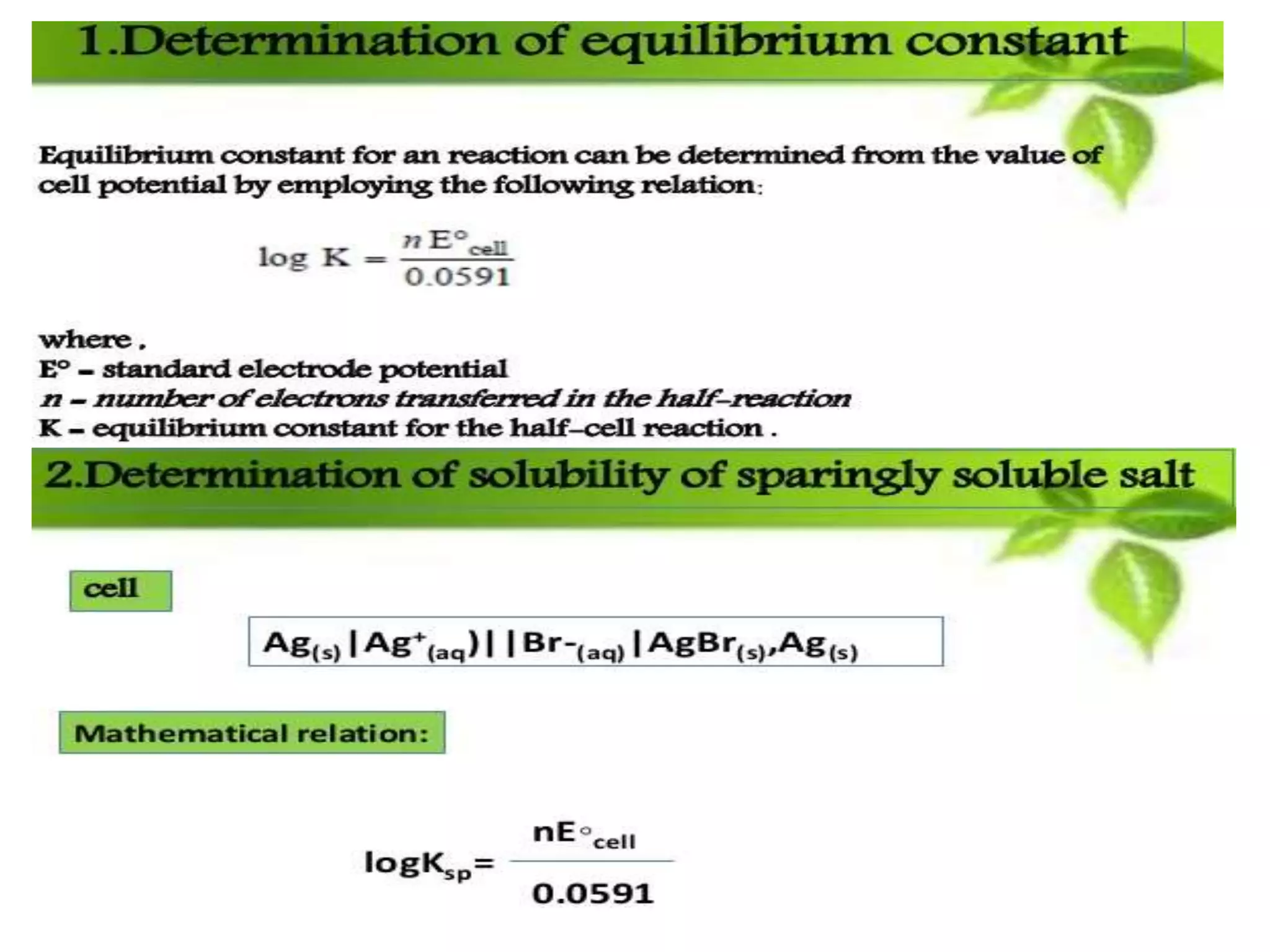
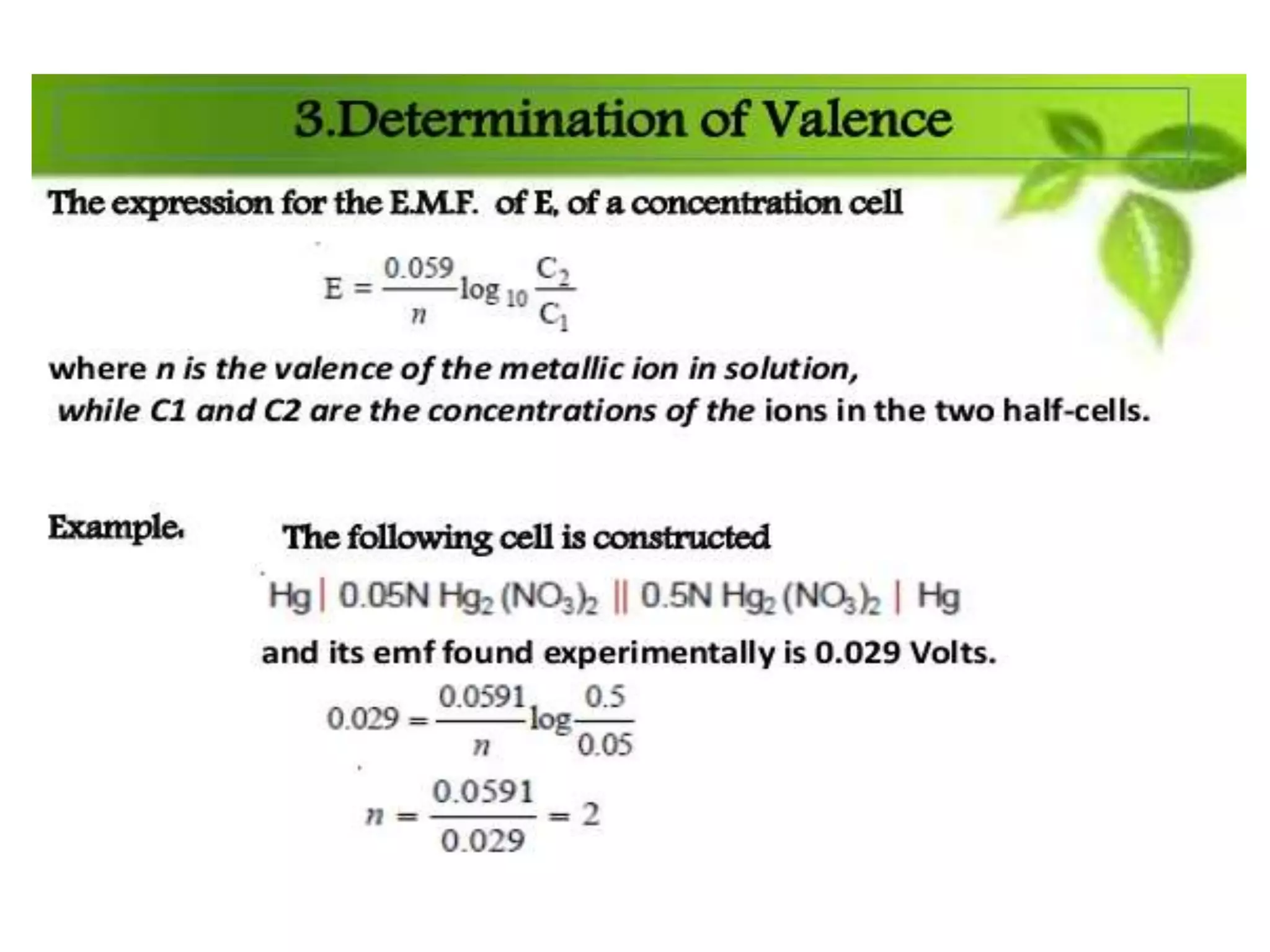
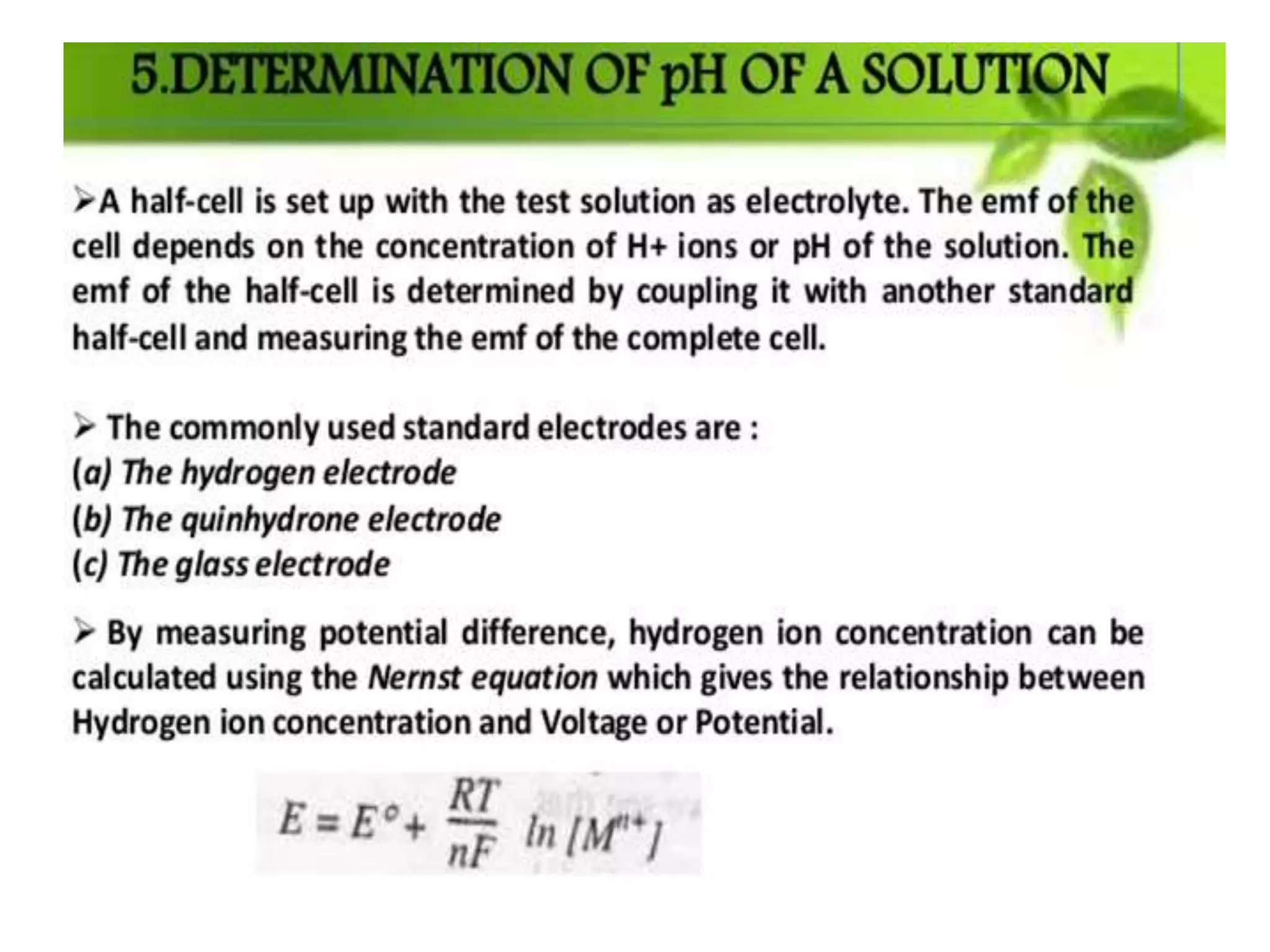
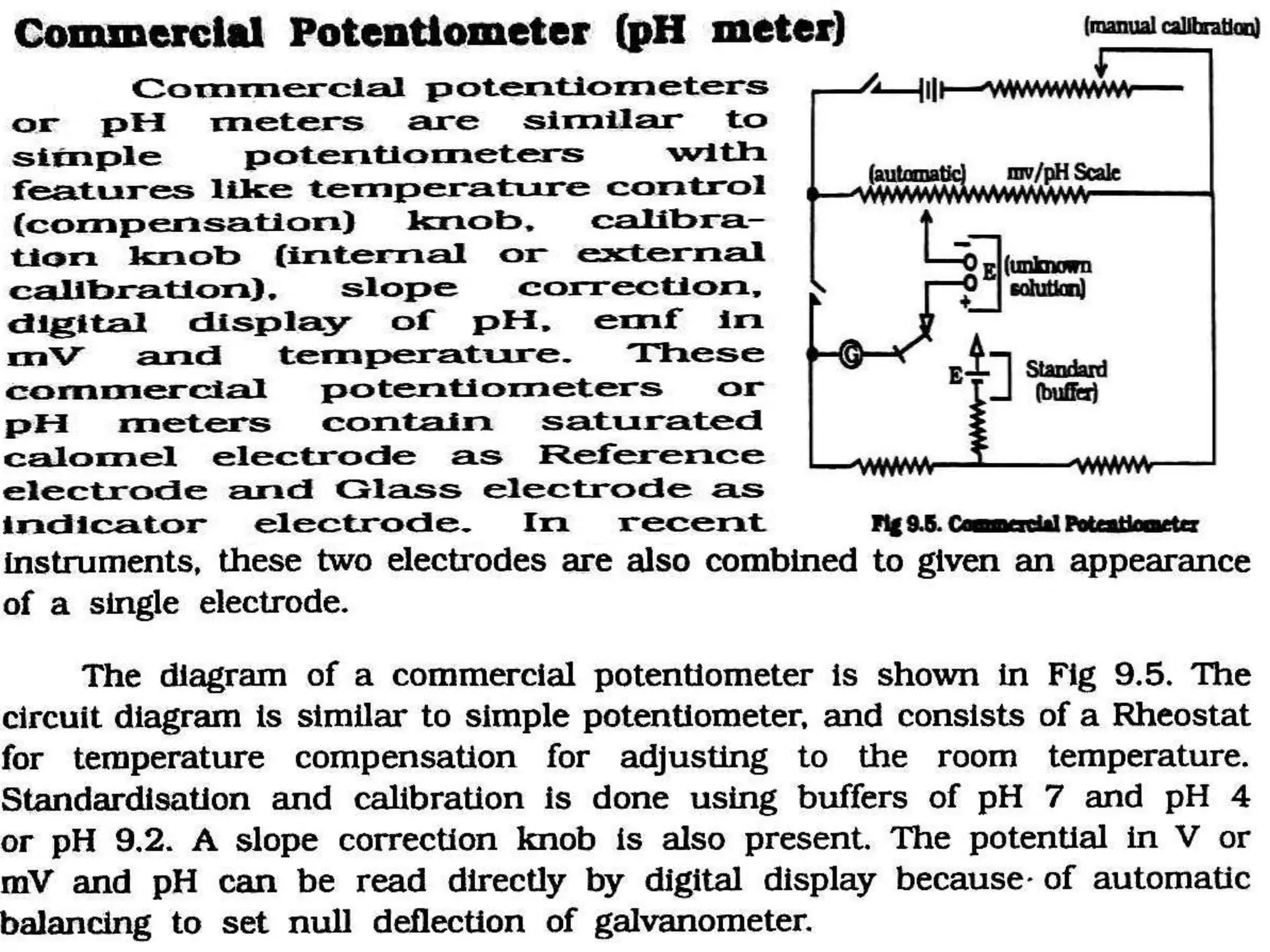
The document discusses pH titration curves for different acid-base reactions. It explains that the equivalence point is when the reactants are mixed in the correct proportions according to the balanced chemical equation, while the end point is when the indicator changes color. Common titration curves are shown for strong acid-strong base, strong acid-weak base, weak acid-strong base, and weak acid-weak base reactions. More complex curves involving carbonate and oxalic acid are also described. The document concludes by discussing how derivative curves can help determine the end point, pH, and electromotive force (emf) of a titration.