IB Chemistry on Acid Base Buffers
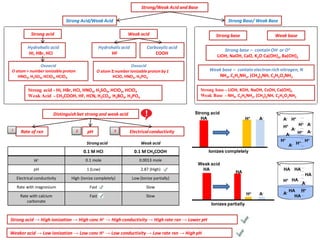
- 1. Strong/Weak Acid and Base Strong Acid/Weak Acid Strong acid - HI, HBr, HCI, HNO3, H2SO4, HCIO3, HCIO4 Weak Acid - CH3COOH, HF, HCN, H2CO3, H3BO3, H3PO4 Strong Base/ Weak Base Strong base - LiOH, KOH, NaOH, CsOH, Ca(OH)2 Weak Base - NH3, C2H5NH2, (CH3)2NH, C3H5O2NH2 Distinguishbet strong and weak acid ElectricalconductivityRate of rxn pH Strongacid Strong acid → High ionization → High conc H+ → High conductivity→ High rate rxn → Lower pH Strong acid Oxoacid O atom > number ionizable proton HNO3, H2SO4, HCIO3,HCIO4 Hydrohalicacid HI, HBr, HCI Weak acid Hydrohalicacid HF Oxoacid O atom ≥ number ionizable protonby 1 HCIO, HNO2, H3PO4 Carboxylicacid COOH Strong base – containOH- or O2- LiOH, NaOH, CaO, K2O Ca(OH)2, Ba(OH)2 Weak base – contain electronrich nitrogen, N NH3, C2H5NH2, (CH3)2NH, C3H5O2NH2 Strong base Weak base 1 2 3 Weak acid 0.1 M HCI 0.1 M CH3COOH H+ 0.1 mole 0.0013 mole pH 1 (Low) 2.87 (High) Electrical conductivity High (Ionize completely) Low (Ionize partially) Rate with magnesium Fast Slow Rate with calcium carbonate Fast Slow Weaker acid → Low ionization → Low conc H+ → Low conductivity→ Low rate rxn → High pH Strong acid HA A-H+ H+ H+ H+ H+ H+ H+ H+A- A- A- A- A- A- Ionizes completely Weak acid HA HA H+ A- H+ H+ A- A- HA HA HA HA HA HA Ionizes partially
- 2. Easier using pH scale than Conc [H+] • Conc H+ increase 10x from 0.0001(10-4) to 0.001(10-3) - pH change by 1 unit from pH 4 to 3 • pH 3 is (10x) more acidic than pH 4 • 1 unit change in pH is 10 fold change in Conc [H+] Conc OH- increase ↑ by 10x pH increase ↑ by 1 unit pOH with Conc OH- pOH = -log [OH- ] [OH- ] = 0.0000001M pOH = -log [0.0000001] pOH = -log1010-7 pOH = 7 pH + pOH = 14 pH + 7 = 14 pH = 7 (Neutral) pH with Conc H+ pH = -log [H+ ] [H+ ] = 0.0000001M pH = -log [0.0000001] pH = -log1010-7 pH = 7 (Neutral) Conc H+ increase ↑ by 10x pH decrease ↓ by 1 unit pH measurement of Acidity of solution • pH is the measureof acidity of solutionin logarithmicscale • pH = powerof hydrogenor minuslogarithmto base ten of hydrogenion concentration ← Acidic – pH < 7 Alkaline – pH > 7 → pOH with Conc OH- pOH = -log [OH- ] [OH- ] = 0.1M pOH = -log[0.1] pOH = 1 pH + pOH = 14 pH + 1 = 14 pH = 13 (Alkaline) pH with Conc H+ pH = -log [H+ ] [H+ ] = 0.01M pH = -log [0.01] pH = -log1010-2 pH = 2 (Acidic) Easier pH scaleConc H+
- 3. Formula for acid/basecalculation [OH-][H+] Kw = [H+] x [OH-] = 1 x 10-14 [OH-] = 10-pOHpOH = -lg [OH-] pOHpH pH = -lg [H+] [H+] = 10-pH pH + pOH = 14 Formula for acid/basecalculation DissociationConstant for Weak Acid pH = -log10[H+] pOH = -log10[OH-] pH + pOH = 14 pH + pOH = pKw Kw = [H+][OH-] Ka x Kb = Kw Ka x Kb = 1 x 10-14 pKa = - lg10Ka pKb = - lg10Kb pKa + pKb = pKw pKa + pKb = 14 AHHA HA AH Ka HCOOCHCOOHCH 33 COOHCH H COOHCH HCOOCH Ka 3 2 3 3 DissociationConstant for Weak Base OHBHOHB 2 B OHBH Kb OHNHOHNH 423 3 2 3 4 NH OH NH OHNH Kb OHCOOCHOHCOOHCH 3323 OHCOOCHOHCOOHCH 3323 COOHCH OHCOOCH Ka 3 33 OHCOOHCHOHCOOCH 323 COOCH OHCOOHCH Kb 3 3 Derive Ka x Kb = Kw Relationship bet Weak acid and its conjugate base Weak acid Conjugate Base COOCH OHCOOHCH COOHCH OHCOOCH 3 3 3 33 OHOH COOCH OHCOOHCH COOHCH OHCOOCH 3 3 3 3 33 wba KKK
- 4. Formula for acid/basecalculation Ka /Kb measureequilibriumposition Ka/Kb large ↑ – ↑ dissociation– shift to right – favour product Ka/Kb large ↑ – pKa /pKb small ↓ – Strongeracid/base Strongacid Large ↑ Ka Weak acid Small ↓ Ka Strongbase Large ↑ Kb Weak base Small ↓Kb ↑ Ka → ↓ pKa Ka /Kb measureequilibriumposition Ka /Kb small ↓ – ↓ dissociation– shift to left – reactant favour Ka /Kb small ↓ – pKa /pKb high ↑– Weak acid/base ↑ Kb → ↓ pKb ↓ Ka → ↑ pKa ↓ Kb →↑ pKb For weak acid/ base CIHHCI OHNHOHNH 423 Shift right Shift left CH3COOH + H2O ↔ CH3COO- + H3O+ CH3COOH CH3COO-CH3COOH ↔ CH3COO- Strong Acid Weak conjugate BaseConjugate acid base pair Small dissociation constant Strong Acid Weak base ba KK / Strongacid Strongbase
- 5. Formula for acid/basecalculation [OH-][H+] Kw = [H+] x [OH-] = 1 x 10-14 [OH-] = 10-pOHpOH = -lg [OH-] pOHpH pH = -lg [H+] [H+] = 10-pH pH + pOH = 14 Formula for acid/basecalculation DissociationConstant for Weak Acid pH = -log10[H+] pOH = -log10[OH-] pH + pOH = 14 pH + pOH = pKw Kw = [H+][OH-] Ka x Kb = Kw Ka x Kb = 1 x 10-14 pKa = - lg10Ka pKb = - lg10Kb pKa + pKb = pKw pKa + pKb = 14 AHHA HA AH Ka HCOOCHCOOHCH 33 COOHCH H COOHCH HCOOCH Ka 3 2 3 3 DissociationConstant for Weak Base OHBHOHB 2 B OHBH Kb OHNHOHNH 423 3 2 3 4 NH OH NH OHNH Kb Dissociatepartially ↔ used Weak acid/base Ka /Kb value pKa /pKb value easier! Click here weak acid dissociation Click here weak acid dissociation Click here CH3COOH dissociation Click here strong acid ionization Weak acid/base Animation
- 6. NH3 ↔ NH4 + Buffer Solution Acid part Neutralize each other Salt part Base part - NH3(weakbase) + NH4CI (salt) - NH3 + H2O ↔ NH4 + + OH− → NH3 moleculeneutralise added H+ - NH4CI → NH4 + + CI− → NH4 + neutralise added OH− - Effectivebuffer equal amt weak base NH3 and conjugate acid NH4 + Acidic Buffer Basic Buffer Resist a change in pH when small amt acid/base is added. CH3COOH + H2O ↔ CH3COO- + H3O+ Acidic Buffer - weak acid and its salt/conjugatebase CH3COOH ↔ CH3COO- Conjugate acid base pair CH3COOH CH3COO- Weak Acid Conjugate Base BUFFER Dissociate fully HCOOCHCOOHCH 33 COOHCH3 COONaCH3 NaCOOCHCOONaCH 33 Dissociate partially - CH3COOH (weakacid) + CH3COONa (salt) - CH3COOH ↔ CH3COO- + H+ → CH3COOH neutraliseadded OH− - CH3COONa → CH3COO- + Na+ → CH3COO- neutraliseadded H+ - Effectivebuffer equal amt weak acid CH3COOH and base CH3COO- COOHCH3 COOCH3 BUFFER Add acid H+Add alkaline OH- Neutralize each other Basic buffer - weak base and its salt/conjugateacid OHNHOHNH 423 NH3 + H2O ↔ NH4 + + OH- NH3 Weak Base NH4 + Conjugate acid CINH43NH BUFFER Conjugate acid base pair Add acid H+ Add alkaline OH- Neutralize each other Neutralize each other Dissociate partially CINHCINH 44 3NH 4NH Base part Salt part Acid part Dissociate fully BUFFER
- 7. How to prepareacidic/ basic buffer Acid Dissociationconstant CH3COOH + H2O ↔ CH3COO- + H3O+ Ka = (CH3COO- ) (H3O+ ) (CH3COOH) -lgKa = -lgH+ -lg (CH3COO-) (CH3COOH) -lgH+ = -lg Ka + lg (CH3COO-) (CH3COOH) pH = pKa + lg (CH3COO-) (CH3COOH) Acidic BufferFormula • Mixture Weak acid + Salt/Conjugatebase • CH3COOH ↔ CH3COO- + H+ (dissociate partially) • CH3COONa → CH3COO- + Na+ (dissociatefully) Basic BufferFormula • Mixture Weak base + Salt/Conjugateacid • NH3 + H2O ↔ NH4 + + OH_ (dissociate partially) • NH4CI → NH4 + + CI_ (dissociate fully) pH = pKa - lg (acid) (salt) pH = pKa + lg (salt) (acid) Base Dissociationconstant NH3 + H2O ↔ NH4 + + OH- Kb = (NH4 + ) (OH- ) (NH3) -lgKb = -lgOH- -lg (NH4 +) (NH3) -lgOH- = -lgKb + lg (NH4 +) (NH3) pOH = pKb + lg (NH4 +) (NH3) pOH = pKb + lg (salt) (base) pOH = pKb - lg (base) (salt) Basic BufferAcidic Buffer salt salt acid base Henderson Hasselbalch Equation multiply -lg both sides Henderson Hasselbalch Equation
- 8. Basic Buffer PreparationAcidic Buffer Preparation Prepare Acidic Buffer pH = 5.2 • Choose pKa acid closest to pH 5.2 • pKa = 4.74 (ethanoic acid) chosen • pH = pKa -lg [acid] [salt] • 5.2 = 4.74 – lg [acid] [salt] • [acid] = 0.35 [salt] Ratio of [acid] = 0.35 [salt] Use same conc acid/salt but different vol ratio • 1M, 35ml (acid) = 0.35 or 0.1M, 35ml (acid) = 0.35 1M, 100ml (salt) 0.1M, 100ml (salt) Use same vol acid/salt but different conc ratio • 3.5M, 10ml (acid) = 0.35 or 0.35M, 10ml (acid) = 0.35 10M, 10ml (salt) 1M, 10ml (salt) Buffer capacity • Adding water will not change the pH of acidic buffer • Ratio of acid/salt still the same • Ka acid remain same Prepare Basic Buffer pH = 9.5 or pOH = 4.5 • Choose pKb base closest to pOH = 4.5 • pKb = 4.74 (NH3) chosen • pOH = pKb -lg [base] [salt] • 4.5 = 4.74 – lg [base] [salt] • [base] = 1.74 [salt] Ratio of [base] = 1.74 [salt] Use same conc base/salt but different vol ratio • 1M, 174ml (base) = 1.74 or 0.1M, 174ml (base) = 1.74 1M, 100ml (salt) 0.1M, 100ml (salt) Use same vol base/salt but different conc ratio • 1.74M, 10ml (base) = 1.74 or 0.174M, 10ml (base) = 1.74 1M, 10ml (salt) 0.1M, 10ml (salt) Buffer capacity • Adding water will not change the pH of basic buffer • Ratio of base/salt still the same • Kb base remainsame Buffer solution Buffer Preparation 1 1 2 2 3 Use fix vol, 1dm3 and use differentmole ratio (Acid/salt) • 0.35 mole acid + 1 mole salt to 1 dm3 solvent= 0.35 Use fix vol, 1dm3 and use differentmole ratio (base/salt) • 1.74 mole base + 1 mole salt to 1 dm3 solvent= 1.74 3 3 ways to prepare buffer 3 ways to prepare buffer
- 9. Basic Buffer PreparationAcidic Buffer Preparation Prepare Acidic Buffer pH = 5.2 • Choose pKa acid closest to pH 5.2 • pKa = 4.74 (ethanoic acid) chosen • pH = pKa -lg [acid] [salt] • 5.2 = 4.74 – lg [acid] [salt] • [acid] = 0.35 [salt] Ratio of [acid] = 0.35 [salt] Use same conc acid/salt but different vol ratio BufferA BufferB • 1M, 35ml (acid) = 0.35 or 0.1M, 35ml (acid) = 0.35 1M, 100ml (salt) 0.1M, 100ml (salt) Prepare Basic Buffer pH = 9.5 or pOH = 4.5 • Choose pKb base closest to pOH = 4.5 • pKb = 4.74 (NH3) chosen • pOH = pKb -lg [base] [salt] • 4.5 = 4.74 – lg [base] [salt] • [base] = 1.74 [salt] Ratio of [base] = 1.74 [salt] Use same conc base/salt but different vol ratio Buffer A Buffer B • 1M, 174ml (base) = 1.74 or 0.1M, 174ml (base) = 1.74 1M, 100ml (salt) 0.1M, 100ml (salt) Buffer solution Buffering Capacity 1 1 1M, 35ml (acid) 1M, 100ml (salt) 0.1M, 35ml (acid) 0.1M, 100ml (salt) BA 1M, 174ml (base) 1M, 100ml (salt) 0.1M, 174ml (base) 0.1M, 100ml (salt) BA Buffer A > Buffer B Stronger buffering capacity • Amt of acid/salt higher to neutralise added H+ or OH- • Ratio acid/salt same, pH buffer same but buffering capacity diff • Higher buffer conc – Higher buffering capacity Buffer A > BufferB Stronger buffering capacity • Amt of base/salt higher to neutralise added H+ or OH- • Ratio acid/salt same, pH buffer same but buffering capacity diff • Higher buffer conc – Higher buffering capacity Which has greater buffering capacity? Which has greater buffering capacity?
- 10. Basic Buffer PreparationAcidic Buffer Preparation Prepare Acidic Buffer pH = 5.2 • Choose pKa acid closest to pH 5.2 • pKa = 4.74 (ethanoic acid) chosen • pH = pKa -lg [acid] [salt] • 5.2 = 4.74 – lg [acid] [salt] • [acid] = 0.35 [salt] Ratio of [acid] = 0.35 [salt] Prepare Basic Buffer at pH = 9.5 or pOH = 4.5 • Choose pKb base closest to pOH = 4.5 • pKb = 4.74 (NH3) chosen • pOH = pKb -lg [base] [salt] • 4.5 = 4.74 – lg [base] [salt] • [base] = 1.74 [salt] Ratio of [base] = 1.74 [salt] Buffer solution Buffering Capacity 2 2 3.5M, 10ml (acid) 10M, 10ml (salt) 0.35M, 10ml (acid) 1M, 10ml (salt) BA 1.74M, 10ml (base) 1M, 10ml (salt) 0.174M, 10ml (base) 0.1M, 10ml (salt) BA Use same vol acid/salt but different conc ratio BufferA BufferB • 3.5M, 10ml (acid) = 0.35 or 0.35M, 10ml (acid) = 0.35 10M, 10ml (salt) 1M, 10ml (salt) Use same vol base/salt but different conc ratio BufferA BufferB • 1.74M, 10ml (base) = 1.74 or 0.174M, 10ml (base) = 1.74 1M, 10ml (salt) 0.10M, 10ml (salt) Which has greater buffering capacity? Which has greater buffering capacity? Buffer A > Buffer B Stronger buffering capacity • Amt of acid/salt higher to neutralise added H+ or OH- • Ratio acid/salt same, pH buffer same but buffering capacity diff • Higher buffer conc – Higher buffering capacity Buffer A > BufferB Stronger buffering capacity • Amt of base/salt higher to neutralise added H+ or OH- • Ratio acid/salt same, pH buffer same but buffering capacity diff • Higher buffer conc – Higher buffering capacity
- 11. Basic Buffer PreparationAcidic Buffer Preparation Buffer solution Buffering Capacity 3 3 0.35mol (acid ) 1mol (salt) 0.035mol (acid) 0.10mol (salt) BA 1.74mol (base) 1mol (salt) 0.174mol (base) 0.1mol (salt) BA Use fix vol, 1dm3 but diff mole ratio (acid/salt) Buffer A Buffer B • 0.35mol (acid) = 0.35 or 0.035mol (acid) = 0.35 1mol (salt) 0.1mol (salt) 1dm3 1dm3 1dm3 1dm3 Use fix vol, 1dm3 but diff mole ratio (base/salt) Buffer A Buffer B • 1.74mol (base) = 1.74 or 0.174mol (base) = 1.74 1mol (salt) 0.1mol (salt) Which has greater buffering capacity? Which has greater buffering capacity? Prepare Acidic Buffer pH = 5.2 • Choose pKa acid closest to pH 5.2 • pKa = 4.74 (ethanoic acid) chosen • pH = pKa -lg [acid] [salt] • 5.2 = 4.74 – lg [acid] [salt] • [acid] = 0.35 [salt] Ratio of [acid] = 0.35 [salt] Prepare Basic Buffer at pH = 9.5 or pOH = 4.5 • Choose pKb base closest to pOH = 4.5 • pKb = 4.74 (NH3) chosen • pOH = pKb -lg [base] [salt] • 4.5 = 4.74 – lg [base] [salt] • [base] = 1.74 [salt] Ratio of [base] = 1.74 [salt] Buffer A > Buffer B Stronger buffering capacity • Amt of acid/salt higher to neutralise added H+ or OH- • Ratio acid/salt same, pH buffer same but buffering capacity diff • Higher buffer conc – Higher buffering capacity Buffer A > BufferB Stronger buffering capacity • Amt of base/salt higher to neutralise added H+ or OH- • Ratio acid/salt same, pH buffer same but buffering capacity diff • Higher buffer conc – Higher buffering capacity
- 12. Basic Buffer PreparationAcidic Buffer Preparation Prepare Acidic Buffer at pH = 5.2 • Choose pKa acid closest to pH 5.2 • pKa = 4.74 (ethanoic acid) chosen • pH = pKa -lg [acid] [salt] • 5.2 = 4.74 – lg [acid] [salt] • [acid] = 0.35 [salt] Ratio of [acid] = 0.35 [salt] Prepare Basic Buffer pH = 9.5 or pOH = 4.5 • Choose pKb base closest to pOH = 4.5 • pKb = 4.74 (NH3) chosen • pOH = pKb -lg [base] [salt] • 4.5 = 4.74 – lg [base] [salt] • [base] = 1.74 [salt] Ratio of [base] = 1.74 [salt] Buffer solution Buffering Capacity 4 4 Will pH change by adding water? pH BufferA = pH Buffer B • Same pH • Adding water will not change pH • Amt of acid/salt still the same • Ratio conc acid/salt same, pH buffer same 0.35mol (acid) 1mol (salt ) 0.35mol (acid ) 1mol (salt) BA 1.74mol (base) 1mol (salt) 1.74mol (base) 1mol (salt) BA Same mole ratio (acid/salt)but differenttotal volume Buffer A BufferB • 0.35mol (acid )= 0.35 in 1dm3 or 0.35mol (acid) = 0.35 in 2dm3 1mol (salt) 1mol (salt) 1dm3 2dm3 1dm3 Same mole ratio (base/salt)but differenttotal volume Buffer A BufferB • 1.74mol (base) = 1.74 in 1dm3 or 1.74mol (base) = 1.74 in 2dm3 1mol (salt) 1mol (salt) 2dm3 Add Water Will pH change by adding water? Add Water pH BufferA = pH Buffer B • Same pH • Adding water will not change pH • Amt of acid/salt still the same • Ratio conc acid/salt same, pH buffer same Weaker buffering capacity
- 13. Acidic Buffer PreparationAcidic Buffer Preparation Prepare Acidic Buffer pH = 4.74 • Choose pKa acid closest to pH 4.74 • pKa = 4.74 (ethanoic acid) chosen • pH = pKa -lg [acid] [salt] • 4.74 = 4.74 – lg [acid] [salt] • [acid] = 1.00 [salt] Ratio of [acid] = 1.00 [salt] Buffer solution Buffering Capacity 5 5 Which has greater buffering capacity? Buffer A > Buffer B • Conc ratio [acid]/[salt] = 1 • Bufferhighest buffering capacity when pH = pKa • Conc acid = Conc salt → highest buffering capacity Concentration ratio [acid]/[salt] = 1 1 mol (acid) 1 mol (salt) A 1 mol (salt) B Buffer A > Buffer B • Further conc ratio [acid]/[salt] from 1 Same conc ratio (acid/salt)in 1dm3 Buffer A • 1 mol (acid ) = 1.00 1 mol (salt) 1dm3 1dm3 Prepare Acidic Buffer at pH = 5.2 • Choose pKa acid closest to pH 5.2 • pKa = 4.74 (ethanoic acid) chosen • pH = pKa -lg [acid] [salt] • 5.2 = 4.74 – lg [acid] [salt] • [acid] = 0.35 [salt] Ratio of [acid] = 0.35 [salt] Differentconc ratio (acid/salt)in 1dm3 Buffer B • 0.35mol (acid ) = 0.35 1.00mol (salt) Which has greater buffering capacity? 0.35mol (acid) Concentration ratio [acid]/[salt] ratio < 1 Lower buffering capacity
- 14. No Salt Hydrolysis Presence of ions from salt cause bonds in water to break NEUTRALIZATION HCI + NaOH → NaCI + H2O Neutral salt Strong acid and Strong base NaCI – Ionize - Na+ and CI- ion – Na+ doesn’t cause water hydrolysis - No breaking bond in water. Strong acid and Weak base Weak acid and Strong base HCI + NH4OH → NH4CI + H2O CH3COOH + NaOH → CH3COONa + H2O Acidic salt Basic salt Salt Hydrolysis Salt Hydrolysis No breaking bond in water NH4CI – Ionize - NH4 + and CI- ion - NH4 + cause water hydrolysis - Breaking bond in water NH4 + + H2O ↔ NH3 + H3O+ CH3COONa– Ionize - Na+ and CH3COO- ion - CH3COO- causewater hydrolysis - Breaking bond in water CH3COO- + H2O ↔ CH3COOH + OH- NH4 + (Acid) - NH3 (Conjugate base) lose H+ to produce H+ gain H+ to produce OH- CH3COO- (Base) - CH3COOH (Conjugate acid) NH4 + + H2O → NH3 + H3O+ NH4CI → NH4 + + CI- H3O+ (Acidic) Cation hydrolysis Anion hydrolysis CH3COONa → CH3COO- + Na+ CH3COO- + H2O→ CH3 COOH + OH- OH- (Alkaline) NaCI → Na+ + CI- No H2O hydrolysis H2O (Neutral)
- 15. NEUTRALIZATION Neutral salt Strong acid and Strong base Strong acid and Weak base Weak acid and Strong base Acidic salt Basic salt NH4 + + H2O ↔ NH3 + H3O+ CH3COO- + H2O ↔ CH3COOH + OH- lose H+ to produce H+ gain H+ to produce OH- NH4 + + H2O → NH3 + H3O+ NH4CI → NH4 + + CI- H3O+ (Acidic) Cation hydrolysis Anion hydrolysis CH3COONa → CH3COO- + Na+ CH3COO- + H2O→ CH3 COOH + OH- OH- (Alkaline) NaCI → Na+ + CI- No H2O hydrolysis H2O (Neutral) HCI + NaOH → NaCI + H2O Neutralization Reaction Salt Salt hydrolysis Type salt pH salt Strong acid + Strong base HCI + NaOH NaCI No hydrolysis Neutral salt 7 Strong acid + Weak base HCI + NH3 NH4CI Cation hydrolysis Acidic salt < 7 Weak acid + Strong base CH3COOH + NaOH CH3COONa Anion hydrolysis Basic salt > 7 Weak acid + Weak base CH3COOH + NH3 CH3COONH4 Anion/Cation hydrolysis Depends ? Click here on acidic buffer simulation Click here buffer simulation
- 16. CH3COO- + H2O → CH3 COOH + OH- Salt Hydrolysis Neutralization Reaction Salt Salt hydrolysis Type salt pH salt Strong acid + Strong base HCI + NaOH NaCI No hydrolysis Neutral salt 7 Strong acid + Weak base HCI + NH3 NH4CI Cation hydrolysis Acidic salt < 7 Weak acid + Strong base CH3COOH + NaOH CH3COONa Anion hydrolysis Basic salt > 7 Weak acid + Weak base CH3COOH + NH3 CH3COONH4 Anion/Cation hydrolysis Depends ? Weak acid and Weak base CH3COOH + NH3 → CH3COONH4 Acidicity depend on Ka and Kb Ka > Kb – Acidic – H+ ions produced Kb < Ka – Basic – OH- ions produced Ka = Kb – Neutral – hydrolyzed same extent. CH3COONH4 → CH3COO- + NH4 + NH4 + + H2O → NH3 + H3O+ salt anion cation OH- - Basic H3O+ - AcidicKb Ka Ka = Kb NEUTRAL NH3 + HF → NH4F salt NH4F → NH4 + + F- NH4 + + H2O → NH3 + H3O+ F- + H2O → HF + OH- cation anion Ka H3O+ - Acidic Kb OH- - Basic Acidicity depend on Ka and Kb Ka > Kb – Acidic – H+ ions produced Kb < Ka – Basic – OH- ions produced Ka = Kb – Neutral – hydrolyzed same extent. Kb > Ka BASIC Weak acid + Weak base
- 17. gain H+ to produce OH- - Basiclose H+ to produce H3O+ - Acidic CH3COO- + H2O → CH3 COOH + OH- Dissociation constant Ka and Kb Weak acid and Weak base CH3COOH + NH3 → CH3COONH4 CH3COONH4 → CH3COO- + NH4 + NH4 + + H2O → NH3 + H3O+ salt anion cation OH- - Basic H3O+ - AcidicKb Ka Ka = Kb NEUTRAL NH3 + HF → NH4F salt NH4F → NH4 + + F- NH4 + + H2O → NH3 + H3O+ F- + H2O → HF + OH- cation anion Ka H3O+ - Acidic Kb OH- - Basic Kb > Ka BASIC Amphoteric Ion Ka = 4.7 x 10 -11 Kb = 2.3 x 10 -8 HCO3 - + H2O ↔ H3O+ + CO3 2- HCO3 - + H2O ↔ H2CO3 + OH- Kb > Ka BASIC Solution of HCO3 - - Acidic or alkaline? Solution of H2PO4 - - Acidic or alkaline? H2PO4 - + H2O ↔ HPO4 2- + H3O+ H2PO4 - + H2O ↔ H3PO4 + OH- lose H+ to produce H3O+ - Acidic Ka = 6.2 x 10 -8 gain H+ to produce OH- - Basic Kb = 1.4 x 10 -12 Ka > Kb ACIDIC
- 18. IB QUESTIONS Predict for each salt whether pH is <, >, = 7 1 HCI + Fe(OH)3 → FeCI3 strong acid + weak base → acidic salt HNO3 + NH4OH → NH4NO3 NaNO3 strong acid + weak base → acidic salt H2CO3 + NaOH → Na2CO3 Weak acid + strong base → basic salt NH4NO3 FeCI3 Na2CO3 CH3COOLi KCN HNO3 + NaOH → Na2CO3 strong acid + strong base → neutral salt CH3COOH + LiOH → CH3COOLi HCN + KOH → KCN 2 3 pH < 7 pH > 7pH < 7 Predict for each salt whether pH is <, >, = 7 Weak acid + strong base → basic salt pH > 7pH = 7 Weak acid + strong base → basic salt pH > 7 Deduce the pH of solution 4 5 6 H2SO4 + NH3 → ? H3PO4 + KOH → ? HNO3 + Ba(OH)2 → ?7 8 9 strong acid + weak base → acidic salt pH < 7 Weak acid + strong base → basic salt pH > 7 strong acid + strong base → neutral salt pH = 7
- 19. Acidic BufferCalculation Find pH buffer - 0.20 mol CH3COONa(salt) add to 0.5dm3, 0.10M CH3COOH(acid) Ka = 1.8 x 10-5 Conc CH3COO- =Moles/volume = 0.20/0.5 = 0.40M Click here videos Khan Academy Find conc of CH3COONa(salt) added to 1.0dm3 of 1.0M CH3COOH(acid) Ka = 1.8 x 10-5M, pKa = 4.74 , pH 4.5 Find pH buffer - 0.10M CH3COOH(acid), 0.25M CH3COONa(salt) Ka = 1.8 x 10-5 1st method (formula) 1 Convert Ka to pKa 2nd method (Ka) 2 1st method (formula) Convert Ka to pKa 2nd method (Ka) 3 1st method (formula) Conc salt 2nd method (Ka) Click here explanation from chem guide 14.5 ]25.0[ ]10.0[ lg74.4 ][ ][ lg pH pH salt acid pKpH a 14.5 )102.7lg( )lg( 102.7 10.0 ))(25.0( 108.1 )( ))(( 6 6 5 3 3 pH pH HpH H H COOHCH HCOOCH Ka 34.5 ]40.0[ ]10.0[ lg74.4 ][ ][ lg pH pH salt acid pKpH a 74.4 )108.1lg( lg 108.1 5 5 a a aa a pK pK KpK K 74.4 )108.1lg( lg 108.1 5 5 a a aa a pK pK KpK K MCOOCH COOCH COOHCH HCOOCH Ka 0578.0 0.1 )1016.3)(( 108.1 )( ))(( 3 5 35 3 3 Msalt salt salt salt acid pKpH a 0578.0][ 24.0 ][ ]0.1[ lg ][ ]0.1[ lg74.45.4 ][ ][ lg 34.5 )105.4lg( )lg( 105.4 10.0 ))(40.0( 108.1 )( ))(( 6 6 5 3 3 pH pH HpH H H COOHCH HCOOCH Ka 5 1016.3 )lg(5.4 )lg( H H HpH Conc [H+]
- 20. Find pH buffer - 0.50M NH3 (base), 0.32M NH4CI (salt) Kb = 1.8 x 10-5 Basic BufferCalculation Find pH buffer - 4.28g NH4CI (salt) add to 0.25dm3, 0.50NH3(base) Kb = 1.8 x 10-5 Mole NH4CI = mass/RMM = 4.28 / 53.5 = 0.08 mol Conc NH4CI = moles/vol = 0.08/0.25 = 0.32M 4 1st method (formula) 2nd method (Kb) 1st method (formula) 5 2nd method (Kb) Conc salt Find mass of CH3COONa added to 500ml, 0.10M CH3COOH(acid) pH = 4.5, Ka = 1.8 x 10-5M, pKa = 4.74 Conc CH3COO- = 0.0578M → x RMM (82) → 4.74g in 1000ml 2.37g in 500ml 6 2nd method (Ka)1st method (formula) Click here addition base to buffer Click here addition acid to buffer 45.955.414 55.4 ]32.0[ ]50.0[ lg74.4 ][ ][ lg pH pOH pOH salt base pKpOH b 45.955.414 55.4 )1081.2lg( )lg( 5 pH pOH pOH OHpOH 5 5 3 4 423 1081.2 50.0 ))(32.0( 108.1 )( ))(( OH OH NH OHNH K OHNHOHNH b 45.955.414 55.4 ]32.0[ ]50.0[ lg74.4 ][ ][ lg pH pOH pOH salt base pKpOH b 45.955.414 55.4 )1081.2lg( )lg( 5 pH pOH pOH OHpOH 5 5 3 4 423 1081.2 50.0 ))(32.0( 108.1 )( ))(( OH OH NH OHNH K OHNHOHNH b 0578.0][ 24.0 ][ ]10.0[ lg ][ ]10.0[ lg74.45.4 ][ ][ lg 3 3 3 3 COOCH COOCH COOCH COOCH acid pKpH a 5.4 10 )lg(5.4 )lg( H H HpH MCOOCH COOCH COOHCH HCOOCH K HCOOCHCOOHCH a 0578.0][ )10.0( )10)(( 108.1 )( ))(( 3 5.4 35 3 3 33 Conc [H+]
- 21. Bicarbonate buffering system Click here view buffering Concept Map Buffer pH Proton availability Stable Buffer solution Weak acid ↔ Conjugate base ][ ][ lg salt acid pKpH a pH = -lg[H+] made up of HA ↔ H+ + A- Weak base ↔ Conjugate acid or Buffering capacity highest Buffer formula pH = pKa 1 ][ ][ baseConjugate Acid B + H2O ↔ BH+ + OH- or Ratio of acid base Dilution Add water pH buffer pH will not change Temperature affect pH pH change Basic Bufferingsystem in blood CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3 - Acid base homeostasis - pH blood plasma constant - buffer range 7.0 – 7.45 Increase CO2 – Shift right – More H+ – pH ↓ - Acidic Decrease CO2 – Shift left – Less H+ - pH ↑ - Alkaline H2CO3 ↔ HCO3 - Weak acid Conjugate base Exercise - release lactic acid H+/CO2 HCO3 - – base neutralize added acid Respiratory acidosis (Hypoventilation) Breathing too slowly – More CO2 in blood – pH ↓– Acidic HCO3 - reabsorb/secretion by kidney, neutralize H+ Respiratory alkalosis (Hyperventilation) Breathing too fast – Less CO2 in blood – pH ↑– Alkaline Release of H+ by kidney to reduce pH ↓ HCO3 - secretion by kidney to reduce pH ↓ Altitude Sickness (Hyperventilation) High altitude – [O2] ↓ – Hyperventilate ↑ – Less CO2 blood ↓ - pH ↑ Drug stimulate secretion HCO3 - / increase H+ secretion by kidney
- 22. Click here on pH calculation Video on Acid/Base Click here on pKa /pKb calculation How pH = pOH = 14 derived How Ka x Kb = Kw derived Simulation on Acid/ Base Click here on pH animation Click here to acid/base simulation Click here on weak base simulation Click here strong acid ionization Click here on weak acid dissociation
