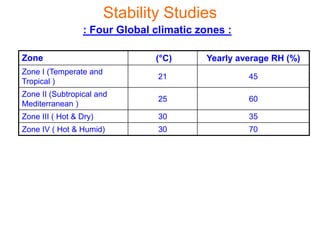
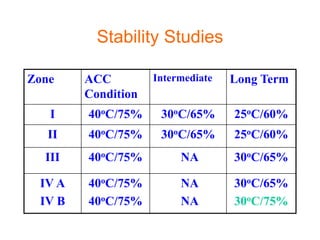
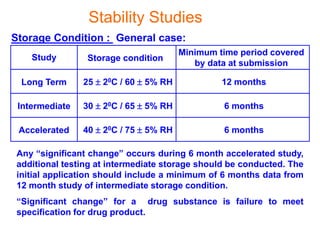
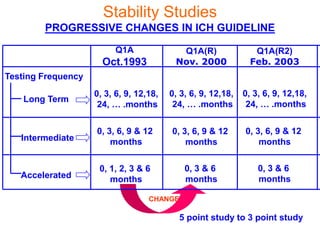
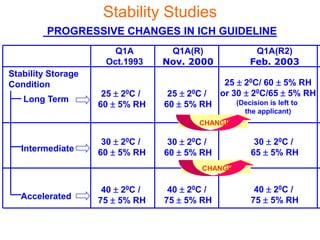
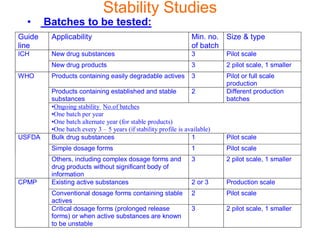
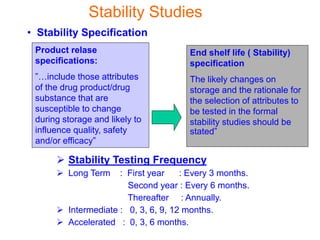
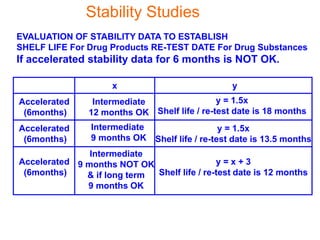
This document discusses stability studies and testing. Stability studies are conducted to provide evidence on how the quality of a drug substance or product varies over time under the influence of environmental factors like temperature, humidity, and light. They are required to recommend storage conditions, establish retest and shelf life periods, review product quality, and meet regulatory requirements. Key aspects covered include guidelines for stability testing, types of studies (long term, intermediate, accelerated), storage conditions, specifications, testing frequency, and requirements for stability protocols, batches, and reports.