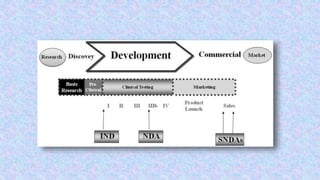
This document discusses supplemental new drug applications (SNDA) which are submitted to the FDA for approval of changes to approved drugs. It defines what types of changes require an SNDA, including manufacturing changes, formulation changes, and labeling changes. It categorizes changes as major, moderate, or minor based on their potential impact on quality, safety, or efficacy. Major changes require prior approval, moderate changes require 30 days' notice, and minor changes are reported annually. Examples are provided for each category of change.