IB Chemistry on Delocalization and Resonance
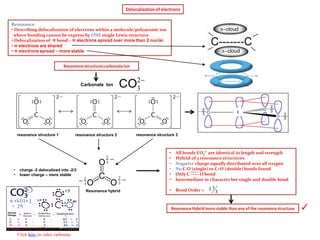
- 1. Delocalizationof electrons Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure • Delocalization of π bond – π electrons spread over more than 2 nuclei • π electrons are shared • π electrons spread – more stable Resonance structurescarbonateion 2 3CO resonance structure 1 resonance structure 2 resonance structure 3 Resonance hybrid • All bonds CO3 2- are identical in length and strength • Hybrid of 3 resonance structures • Negative charge equally distributed over all oxygen • No C-O (single) or C=O (double) bonds found • Only C ----- O bond • Intermediate in character bet single and double bond • Bond Order = 3 11 Carbonate Ion • charge -2 delocalized into -2/3 • lower charge – more stable Click here on video carbonate C Resonance Hybrid more stable than any of the resonancestructure ✓
- 2. Delocalizationof electrons Resonance structuresnitrate ion 3NO resonance structure 1 resonance structure 2 resonance structure 3 resonance hybrid • All bonds NO3 - are identical in length and strength • Hybrid of 3 resonance structures • Negative charge equally distributed over all oxygen • No N-O (single) or N=O (double) bonds found • Only N ----- O bond • Intermediate in character bet single and double bond • Bond Order = 3 11 Nitrate Ion • charge of -1 delocalized into -1/3 • lower charge – more stable Click here to view video Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure • Delocalization of π bond – π electrons spread over more than 2 nuclei • π electrons are shared • π electrons spread – more stable 3 1 3 1 3 1 Resonance Hybrid more stable than any of the resonancestructure ✓
- 3. Delocalizationof electrons Resonance structuresnitrite ion 2NO resonance structure 1 resonance structure 2 resonance hybrid • All bonds NO2 - are identical in length and strength • Hybrid of 2 resonance structures • Negative charge equally distributed over all oxygen • NO N-O (single) or N=O (double) bonds found • Only N ------ O bond • Intermediate in character bet single and double bond • Bond Order = Nitrite Ion • charge of -1 delocalized into -1/2 • lower charge – more stable Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure • Delocalization of π bond – π electrons spread over more than 2 nuclei • π electrons are shared • π electrons spread – more stable Click here video nitrite 2 1 2 1 1.5 Resonance Hybrid more stable than any of the resonancestructure ✓
- 4. Delocalizationof electrons Resonance structuressulfur dioxide 2SO resonance structure 1 resonance structure 2 • All SO2 bonds are identical in length and strength • Hybrid of 2 resonance structures • NO S-O (single) or S=O (double) bonds found • Only S ------O bond • Intermediate in character bet single and double bond • Bond Order = Sulfur Dioxide Click here to view S Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure • Delocalization of π bond – π electrons spread over more than 2 nuclei • π electrons are shared • π electrons spread – more stable resonance structure 3 How about structure 3? resonance hybrid 1.5 Resonance Hybrid more stable than any of the resonancestructure ✓
- 5. Delocalizationof electrons Resonance structuressulfur trioxide resonance structure 1 resonance structure 2 • All SO3 bonds are identical in length and strength • Hybrid of 3 resonance structures • NO S-O (single) or S=O (double) bonds found • Only S ----- O bond • Intermediate in character bet single and double bond • Bond Order = 3 11 Sulfur Trioxide 3SO resonance structure 3 S 120 Click here to view video Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure •Delocalization of π bond – π electrons spread over more than 2 nuclei •π electrons are shared •π electrons spread – more stable resonance structure 4 How about structure 4 ? resonance hybrid Resonance Hybrid more stable than any of the resonancestructure ✓
- 6. Delocalizationof electrons Resonance structuresmethanoate resonance structure 1 resonance structure 2 • All CO bonds are identical in length and strength • Hybrid of 2 resonancestructures • Negative charge equally distributed over oxygen atom • NO C-O (single) or C=O (double) bonds found • Only C ----- O bond • Intermediate character bet single and doublebond • Bond Order = Methanoate ion HCOO Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure • Delocalization of π bond – π electrons spread over more than 2 nuclei • π electrons are shared • π electrons spread – more stable Click here to view resonance hybrid Click here to view Resonance structuresethanoate Ethanoate ion COOCH3 resonance structure 1 resonance structure 2 resonance hybrid HH CH3 2 1 2 1 2 1 2 1 • charge of -1 delocalized into -1/2 • lower charge – more stable 1.5 Resonance Hybrid more stable than any of resonance structure ✓
- 7. Delocalizationof electrons Resonance structuresozone resonance structure 1 resonance structure 2 resonance hybrid • All bonds O-O are identical in length and strength • Hybrid of 2 resonance structures • NO O-O (single) or O=O (double) bonds found • Only O ----- O bond • Intermediate in character bet single and double bond • Bond Order = Ozone 3O Click here on video ozone Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure • Delocalization of π bond – π electrons spread over more than 2 nuclei • π electrons are shared • π electrons spread – more stable • Pale blue gas, polar, dimagnetic • Oxidizing agent • Potent respiratory hazard and pollutant at ground level • Beneficialprevent UV B/C from reaching Earthsurface • Highest ozone level in stratosphere,(10 km and 50 km) UV radiation Ozone at stratosphere strongest radiation 3O O-O Single bond O=O Double bond O=O=O Inter mediate Bond length/pm 148 121 127 Bond enthalpy/kJ mol-1 144 498 364 Bond order 1 2 1.5 ✓ 1.5 Resonance Hybrid more stable than resonancestructure ✓
- 8. Delocalizationof electrons Resonance • Describing delocalization of electrons within a molecule/polyatomic ion where bonding cannot be express by ONE single Lewis structure • Delocalization of π bond – π electrons spread over more than 2 nuclei • π electrons are shared • π electrons spread – more stable Resonance structuresbenzene Benzene 6HC6 resonance structure 1 resonance structure 2 Resonance hybrid • All bonds C6H6 are identical in length/strength • Hybrid of 2 resonance structures • No C-C (single) or C=C (double) bonds found • Only C ----- C bond • Intermediate character bet single/double bond • Bond Order = • Unhybridised p orbital • Delocalizationelectronsabove below plane • sp2 hybridization on carbon center 1.5 Click here to view Delocalized electrons Kekulé structure Cyclohexa- 1,3,5 triene χ ✓ double/single bonds bet them Benzene Hexagonal, planar Resonance Hybrid more stable than resonance structure ✓ Click here to view Kekule
- 9. Resonance/DelocalizationEnergy ΔH cyclohexene = -120kJmol-1 ΔH cyclohexa 1,3 diene = -240kJmol-1 ΔH cyclohexa 1,3,5 triene = -360kJmol-1 ΔH Benzene = -208kJmol-1 Enthalpychangehydrogenation ✓ ✓ χ …… • Benzene lower in energy by 150kJ • More stable due to delocalization of π electrons 150kJ -150 C-C Single bond C=C Double bond C=C Benzene Bond length/pm 154 134 140 Bond enthalpy/kJmol-1 346 614 507 3Evidencefor Benzenestructure 1 2 Click here evidenceagainst Kekule • X ray hit benzene crystal • Interact with electron (electron density map) • X ray diffraction produced • Bond length measured X ray crystallography NO single/double bond detected ✓ ✓ 3 Addition reaction for unsaturated C=C ✓Addition reaction Substitution reaction NO double bond
- 10. FORMAL CHARGE (FC) Tool/Modelfor comparingwhich Lewis structuresis more acceptable Lewis structure SO2 Which is acceptable? Lewis structure SO3 Formal Charge • Treats covalent bond with equal electron distribution no EN diff bet atom • Electronegativeatom has negative while least electronegative atom has positive formal charge. Formula formal charge Click here video formal chargesClick here video formal charges V - valence electrons of atom L – Lone pair electron B - electrons shared in covalent bonds in the molecule ✓ ✓ All resonancestructure contributeto electronic structure. Real structure is combinationof them. Lowest formalcharge (stable), contribute more than less stable structure. Sum of formalcharges must be zero for neutral or equal to charge on ion. L + Formal charge concept
