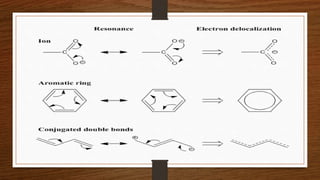
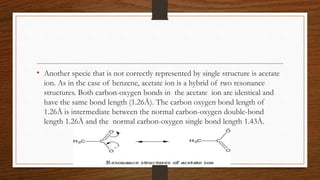
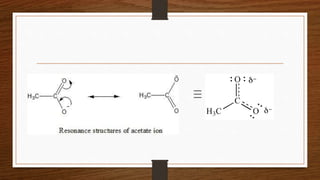
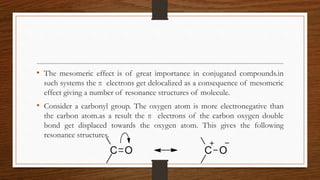
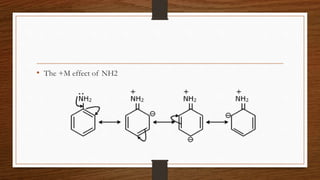
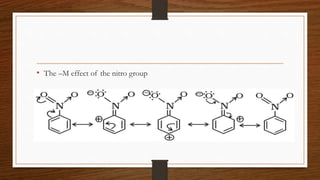
1. Resonance occurs when a molecule can be represented by two or more structures that differ only in electron arrangements, not atom positions.
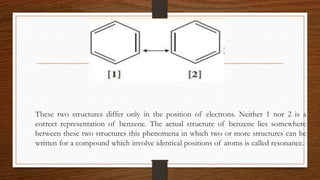
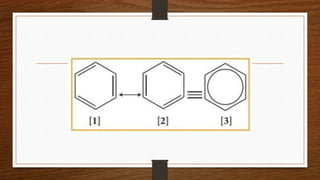
2. The actual structure of molecules like benzene and acetate ion are resonance hybrids that are intermediate between hypothetical resonance structures.
3. Resonance hybrids are more stable than any single contributing resonance structure. The extra stability is called the resonance energy.