Embed presentation
Downloaded 23 times
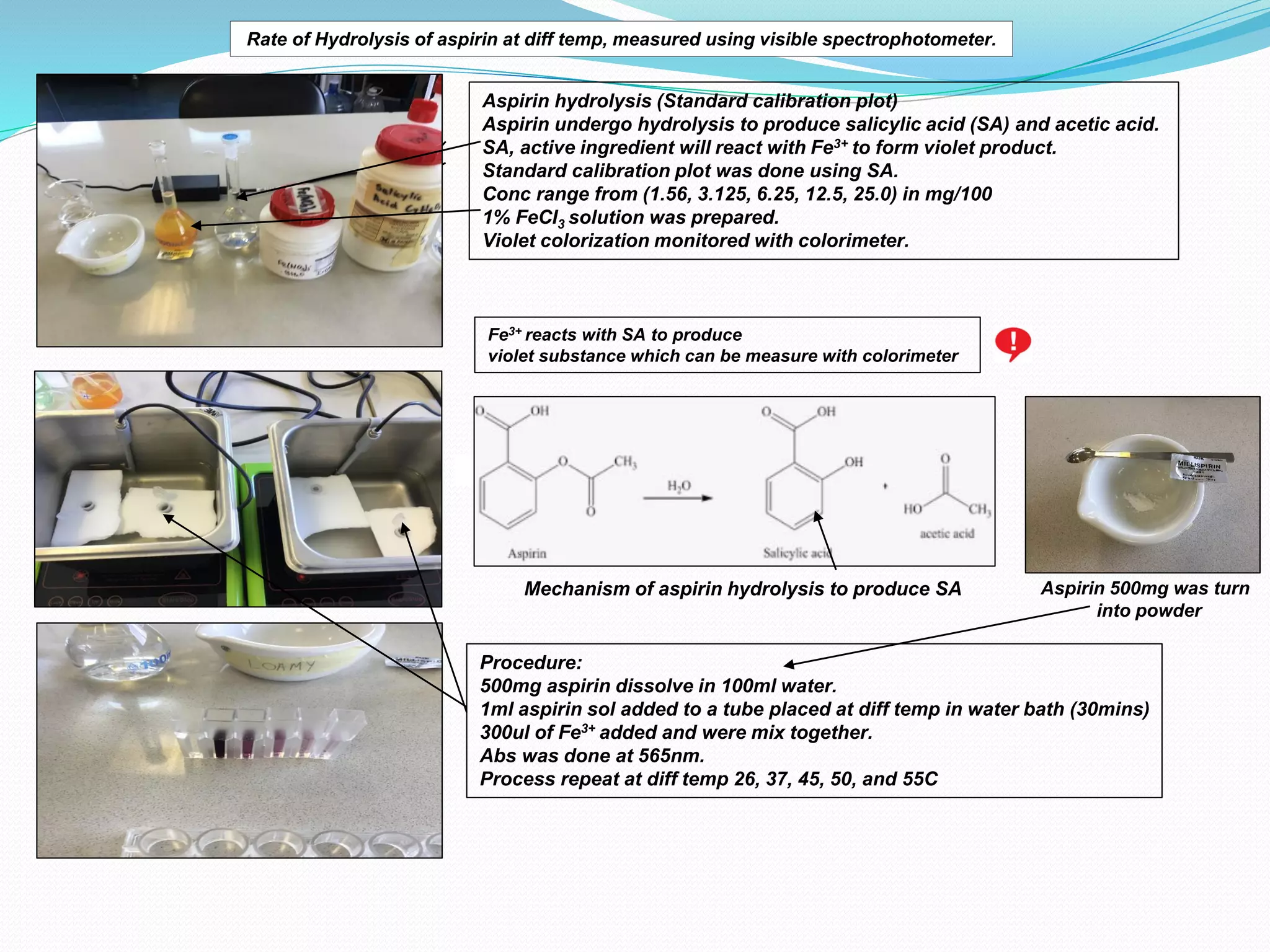
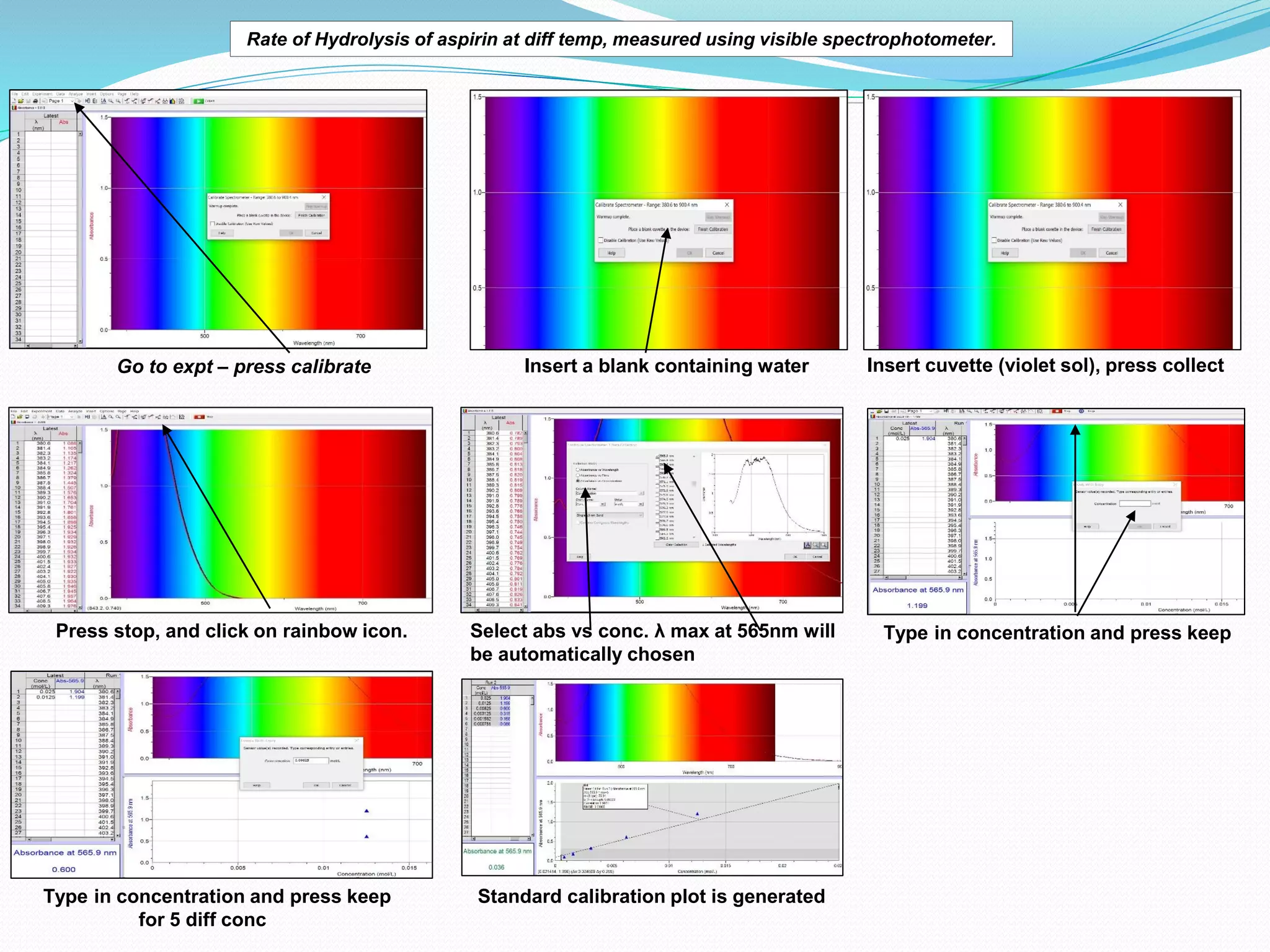
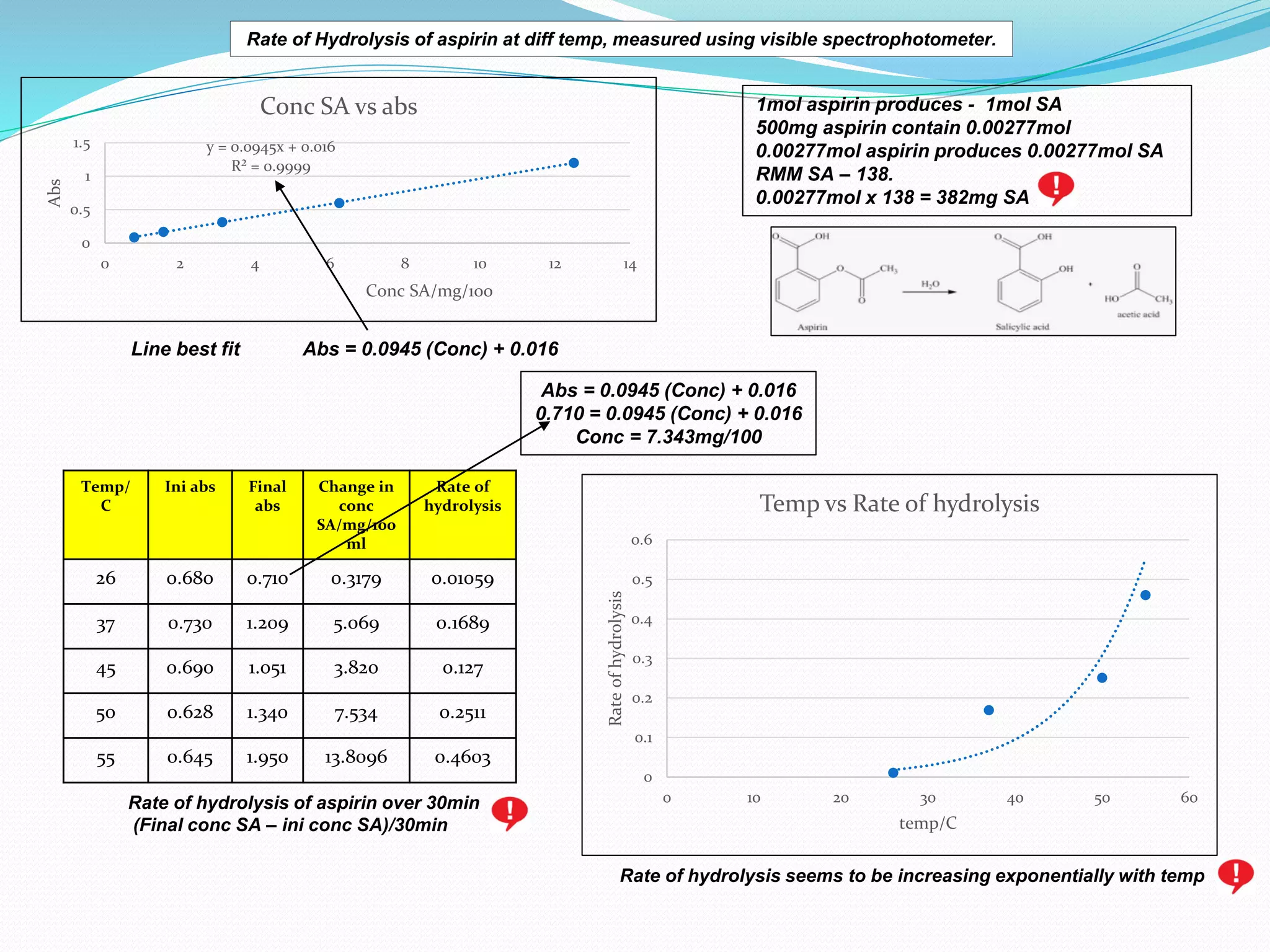

The document details an experiment on the hydrolysis of aspirin, producing salicylic acid (SA) and acetic acid, with the reaction monitored using a colorimeter to measure the formation of a violet product with Fe3+. It presents a standard calibration plot for SA concentrations and outlines the methodology for testing various temperatures on the hydrolysis rate. Results indicate that the rate of hydrolysis increases exponentially with temperature, demonstrating the relationship between temperature and the reaction rate.


