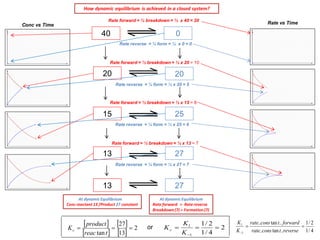
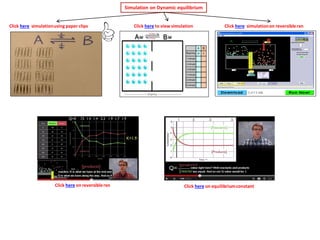
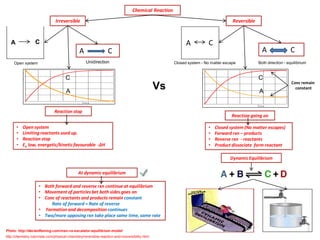
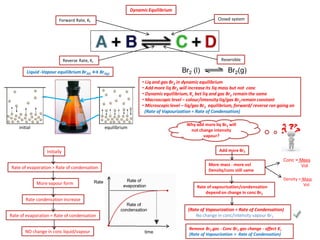
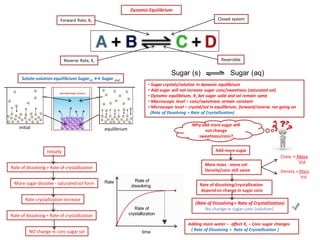
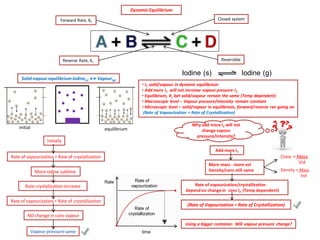
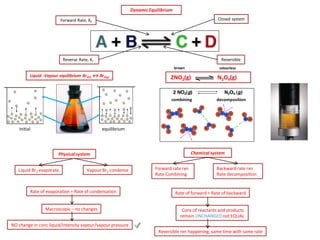
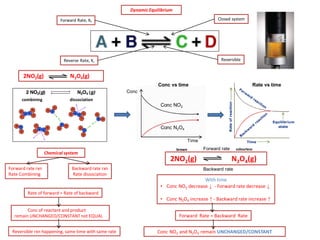
The document discusses dynamic equilibrium in chemical reactions. It explains that in a closed system, reversible reactions proceed in both the forward and reverse directions at the same rate, such that the concentrations of reactants and products remain constant over time. It provides examples of chemical equilibria and how equilibrium is achieved as the reaction progresses through opposing changes in reaction rates and concentrations. Equilibrium constants are also introduced which relate the concentrations of products and reactants at equilibrium.
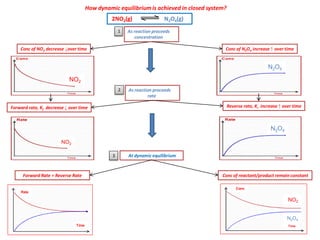
![Dynamic Equilibrium
Reversible (closed system)
Forward Rate, K1 Reverse Rate, K-1
Kc = ratio of molar conc of product (raised to power of their respective stoichiometry coefficient)
to molar conc of reactant (raised to power of their respective stoichiometry coefficient)
Conc of product and reactant
at equilibrium
At Equilibrium
Forward rate = Backward rate
Conc reactants and products remain
CONSTANT/UNCHANGE
Equilibrium Constant Kc
aA(aq) + bB(aq) cC(aq) + dD(aq)
coefficient
Solid/liq not included in Kc
Conc represented by [ ]
K1
K-1
a b
c d
c
A B
C D
K
1
1
K
K
Kc
Equilibrium Constant Kc
express in
Conc vs time Rate vs time
A + B
C + D
Conc
Time
Click here notes on dynamic equilibrium
Excellent Notes
K1 = forward rate constant
K-1 = reverse rate constant](https://image.slidesharecdn.com/equilibriumnewpdf-141113040215-conversion-gate01/85/IB-Chemistry-on-Dynamic-Equilibrium-and-Equilibrium-Constant-8-320.jpg)
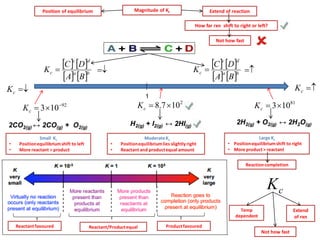
![Equilibrium Constant Kc
a b
c d
c
A B
C D
K
aA(aq) + bB(aq) cC(aq) + dD(aq)
Conc of product and reactant at equilibrium
Equilibrium expression HOMOGENEOUS gaseous rxn
4NH3(g) + 5O2(g) ↔ 4NO(g) + 6H2O(g) N2(g) + 3H2(g) ↔ 2NH3(g)
NH4CI(s) ↔ NH3(g) + HCI(g)
2SO2(g) + O2(g) ↔ 2SO3(g)
5
2
4
3
6
2
4
NH O
NO H O
Kc
3
2
1
2
2
3
N H
NH
Kc
1 1
3 K NH HCI c
0
4
1 1
3
NH CI
NH HCI
Kc
1
2
2
2
2
3
SO O
SO
Kc
Equilibrium expression HETEROGENOUS rxn
CaCO3(s) ↔ CaO(g) + CO2(g)
0
3
1
2
1
CaCO
CaO CO
Kc
1
2
1 K CaO CO c
CH3COOH(l) + C2H5OH(l) ↔ CH3COOC2H5(l) + H2O(l)
1
2 5
1
3
1
2
1
3 2 5
CH COOH C H OH
CH COOC H H O
Kc
Equilibrium expression HOMOGENEOUS liquid rxn
Cu2+
(aq) + 4NH3(aq) ↔ [Cu(NH3)4]2+
4
3
2 1
2
3 4 ( )
Cu NH
Cu NH
Kc
Reactant/product same phase
Reactant/product diff phase
Solid and liq - conc no change
(not included)](https://image.slidesharecdn.com/equilibriumnewpdf-141113040215-conversion-gate01/85/IB-Chemistry-on-Dynamic-Equilibrium-and-Equilibrium-Constant-10-320.jpg)