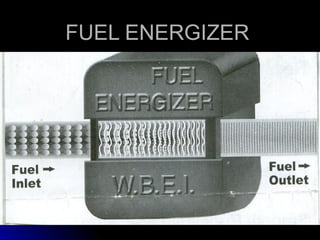
This document discusses hydrocarbon fuels and the role of fuel energizers. It notes that hydrocarbons leave carbon residue during combustion and do not combust until vapourized. This leads to issues like pinging and reduced efficiency. Methane is a major constituent of natural gas and is an important hydrogen source, with most of its energy coming from hydrogen. Hydrogen exists in para and ortho states, with ortho being more reactive. Fuel energizers use neodymium magnets to produce strong magnetic fields, breaking up hydrocarbons to increase surface area and changing hydrogen states to improve combustion. They are installed on fuel lines to provide benefits like increased mileage and reduced emissions.