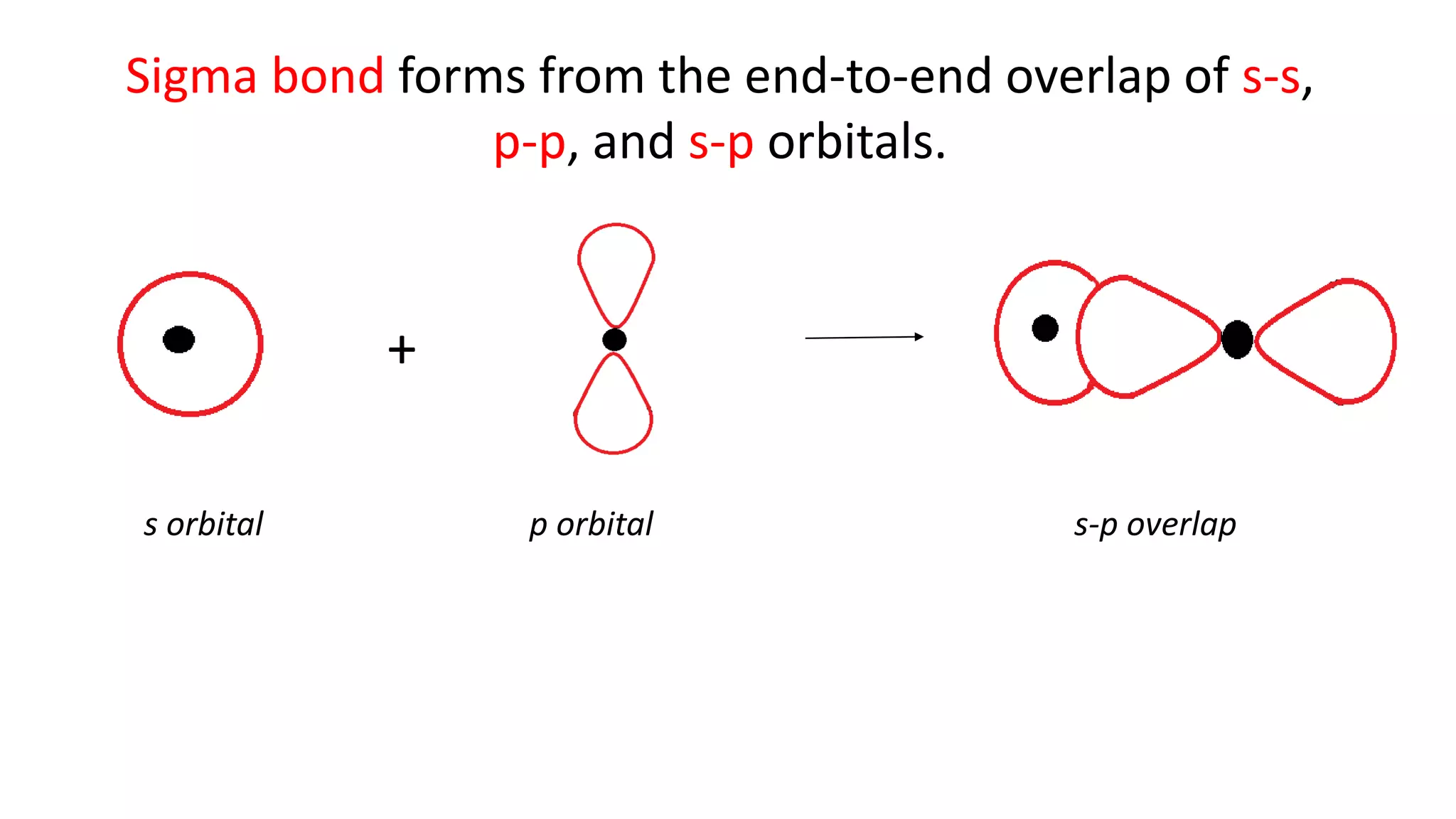
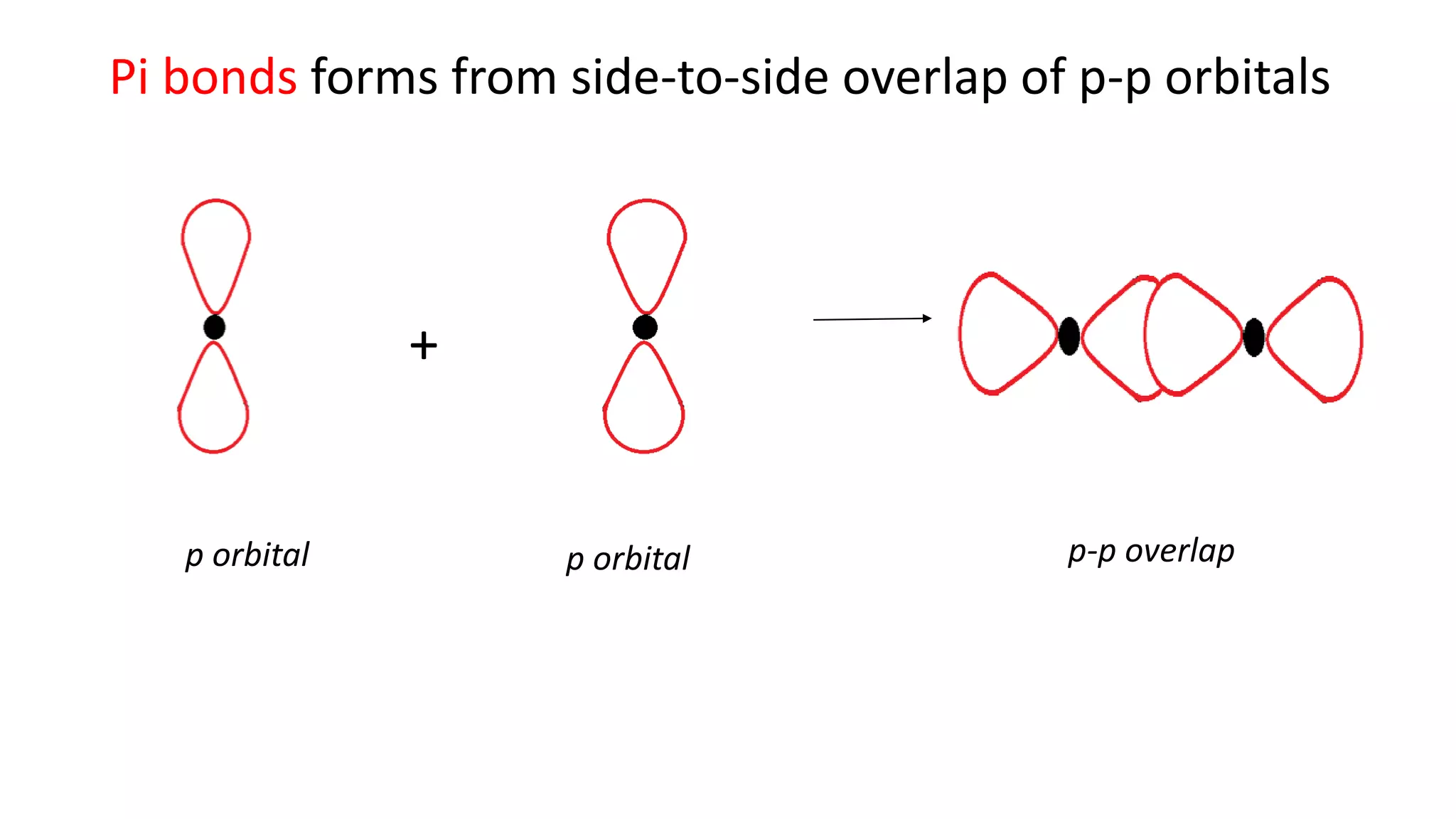
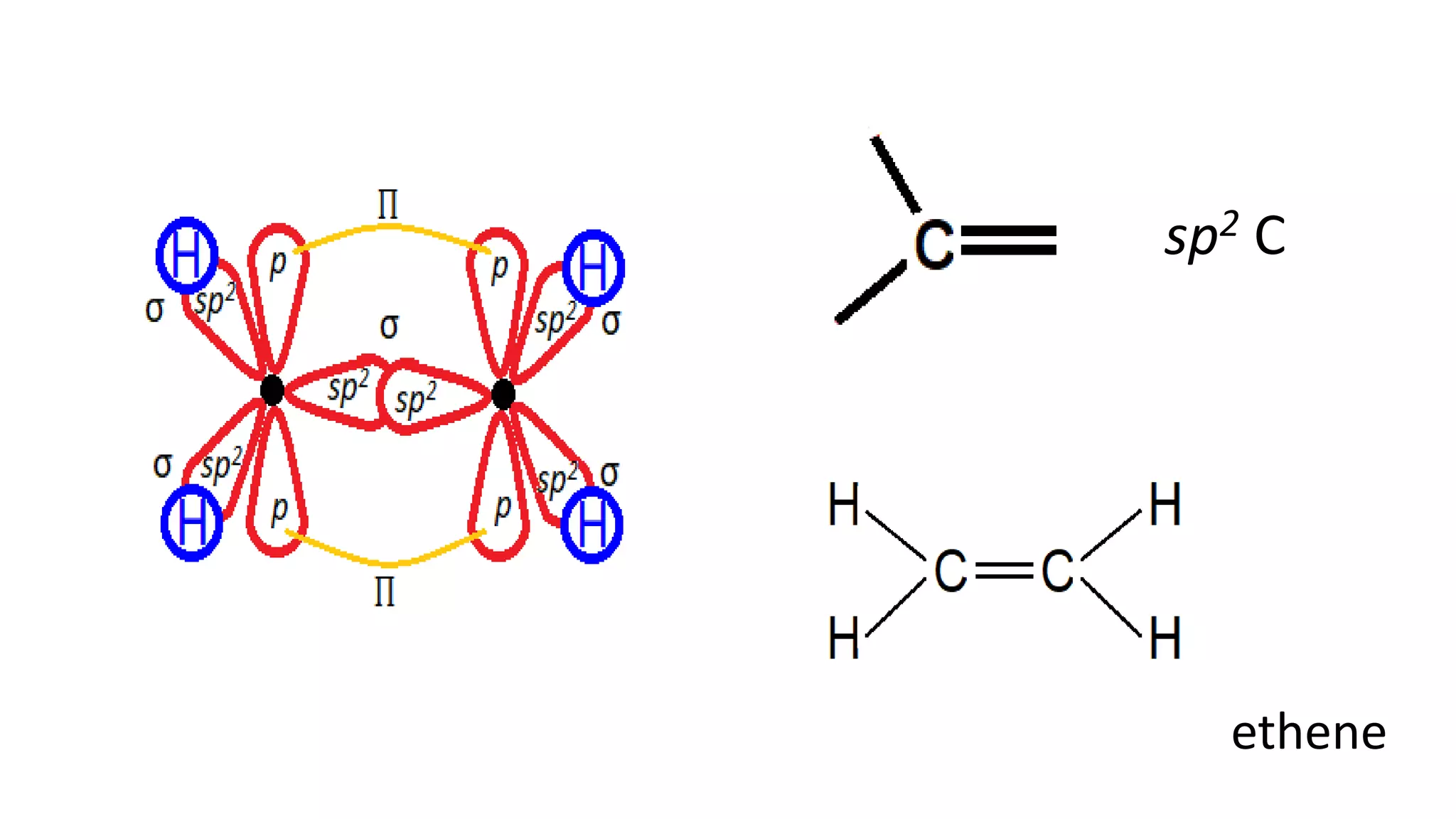
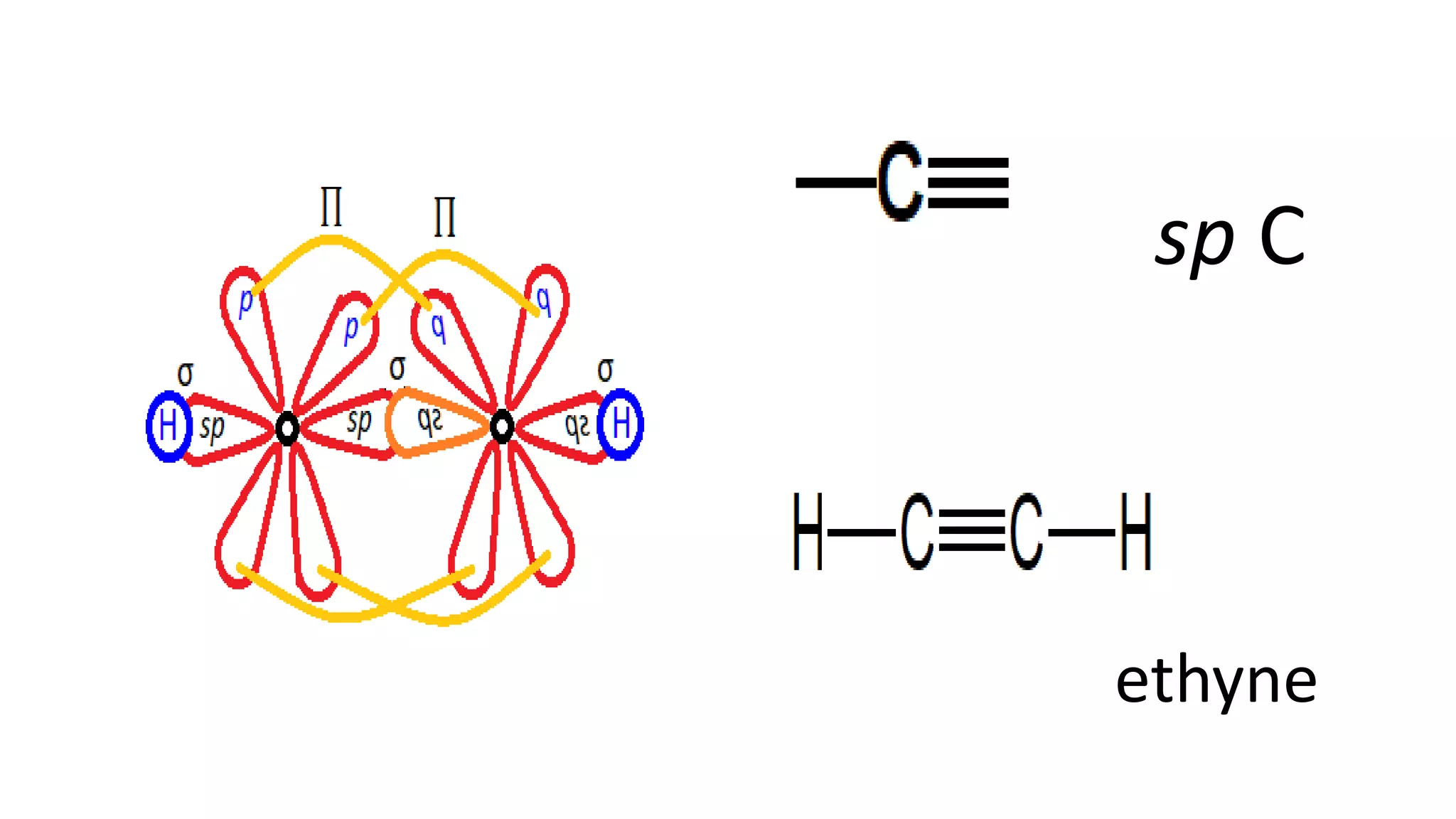
The document explains the bonding and geometry of ethane, ethene, and ethyne in terms of hybridization and types of carbon-carbon bonds. It details how carbon's four valence electrons hybridize into sp3, sp2, or sp arrangements, leading to sigma and pi bonds, and describes the resulting geometries: tetrahedral for ethane, trigonal planar for ethene, and linear for ethyne. The document emphasizes the relationship between hybridization and bond formation, indicating that single, double, and triple bonds arise from different combinations of sigma and pi bonds.