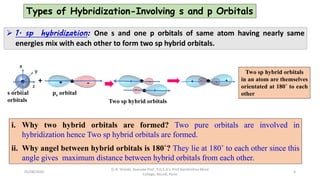
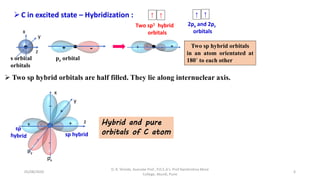
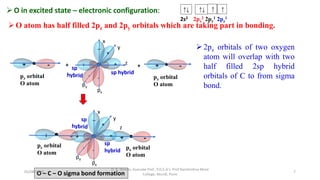
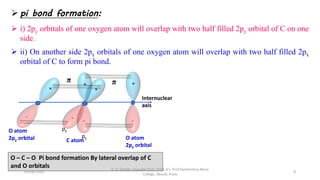
1. The document discusses hybridization in molecules, specifically sp, sp2, and sp3 hybridization.
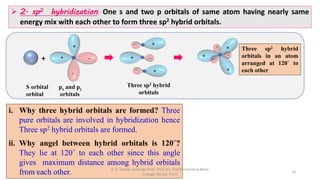
2. Hybridization occurs when atomic orbitals with similar energies mix to form new hybrid orbitals during molecule formation. This explains the actual geometries and bond angles observed in molecules.
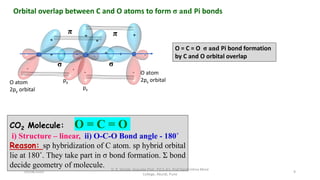
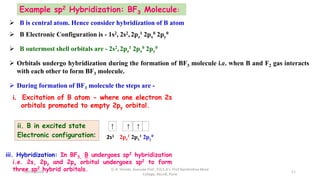
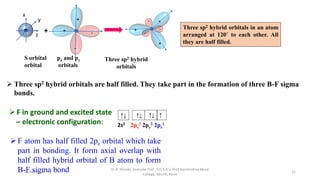
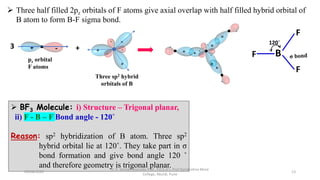
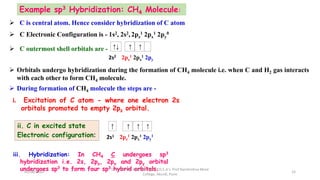
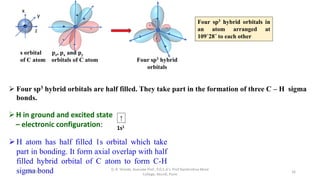
3. Examples of CO2, BF3, and CH4 are provided to illustrate sp, sp2, and sp3 hybridization respectively. The hybrid orbitals formed determine the molecular geometry and bond angles in each case.






![On the basis of hybridization of orbital on the central atom one can explain the
geometry and bond angles of all molecule.
Sr.
No
Pure Orbitals
used for
hybridization
Hybridi-
zation
Geometry Bond
Angle
Example
1 s and p sp linear 180˚
CO2
Ag in [Ag(NH3)2]+
2 s and two p sp2 Trigonal
Planar
120˚ BF3
3
s and three
p
sp3 Tetrahedral 109˚28̎
CH4
NiCl4
Summery
05/08/2020
D. R. Shinde, Asociate Prof., P.D.E.A's. Prof Ramkrishna More
College, Akurdi, Pune
18](https://image.slidesharecdn.com/chemicalbonding-hybridization-1-200805151235/85/Hybridization-1-18-320.jpg)
