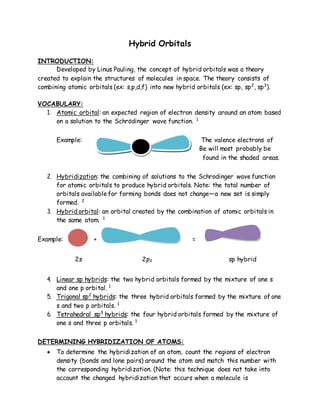
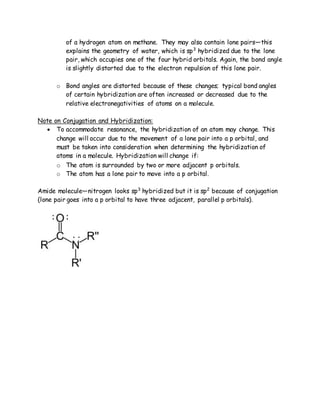
The document explains the concept of hybrid orbitals developed by Linus Pauling, which combines atomic orbitals into hybrid forms (sp, sp2, sp3) to describe molecular structures. It details the determination of hybridization based on regions of electron density around atoms and the resulting molecular geometry influenced by hybridization. Additionally, it addresses how hybridization can change in the presence of conjugation, affecting the accuracy of hybridization assessments.